Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The tip of the iceberg in organic chemistry classes: how do students deal with the invisible?
Nicole
Graulich
Justus-Liebig University Giessen, Institute of Chemistry Education, Heinrich-Buff Ring 58, 35392 Giessen, Germany. E-mail: Nicole.Graulich@org.chemie.uni-giessen.de; Fax: +49-641-9934609; Tel: +49-641-9934600
First published on 17th November 2014
Abstract
Organic chemistry education is one of the youngest research areas among all chemistry related research efforts, and its published scholarly work has become vibrant and diverse over the last 15 years. Research on problem-solving behavior, students' use of the arrow-pushing formalism, the investigation of students' conceptual knowledge and their cognitive skills have shaped our understanding of college students' understanding in organic chemistry classes. This review provides an overview of research efforts focusing on student's perspectives and summarizes the main results and pending questions that may guide subsequent research activities.
Introduction
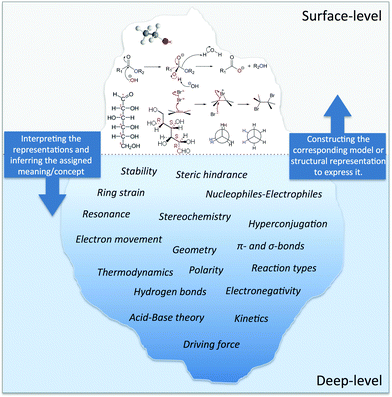
Only one-tenth of an iceberg's volume is above the water; the rest is beneath the surface. You cannot judge the shape or size of the underwater portion by looking at the portion above the surface. To get the whole picture, you have to consider the deeper level or what you can infer from the surface. This analogy of an iceberg could represent the nature of organic chemistry taught in a classroom context (Fig. 1). As Kozma and Russell (1997) stated: “Much of what is chemistry exists at a molecular level and is not accessible to direct perception” (Kozma and Russell, 1997, p. 949). Thus, we build models and concepts about phenomena, like acid–base theories, and use a large catalog of conventions to draw or visualize compounds. By writing a simple molecule like H2O, multiple pieces of chemical information are related to a short sequence of letters and numbers: drawing those pieces two dimensionally conveys the geometry. Additionally, relating electronegativity to the atoms can explain dipolar properties and the hydrogen bonding effect. Hence a large part of the scientific practice in an organic chemistry classroom takes place by using pictorial representations that convey deeper meanings. Small structural changes at a molecular structure can entirely alter the mechanism of a chemical reaction, such as substitution reactions at a tert-butylalcohol and ethanol.Since the ability to make inferences from the surface or structural level is crucial in organic chemistry, the emergent questions ask how students learn to make these deep level connections and what problems they encounter when interpreting chemical representations, proposing mechanisms, or making structure–reactivity judgments. As Goodwin (2008) states, “By deciding which of these concepts apply to the compounds (or intermediates) of a particular transformation, it is often possible to explain, or even to predict, facts about the outcome, mechanism, and rates of the transformation, even when that transformation has never been encountered before” (Goodwin, 2008, p. 126). Understanding the embedded basic concepts in organic chemistry and using this knowledge as a source of prediction are huge challenges for students.
Students' sense-making processes at the symbolic level became the emergent topic in the organic chemistry research community over the last 15 years. This review aims at providing an overview of what is known from current, student-centered research about the nature of college students' understanding in organic chemistry with an emphasis on problems encountered in traditional organic chemistry classes. Thus, the tip of the iceberg highlighted in this review primarily refers to students' understanding of symbolic representations, such as structural formulae and less to macroscopic entities that are more important in laboratory classes.
Four highly interrelated topics central to teaching and learning organic chemistry are at the focus of this review: cognitive skills, problem-solving, conceptual knowledge, and epistemological development. Reviewing the literature of the last 15 years with regard to these main research areas had been difficult, as some studies examined various facets. The organization of the review should thus not be taken as definitive. Teaching initiatives, laboratory studies and curriculum improvements are not discussed in this overview, but teaching implications and an outline of pending research questions are given at the end of each section.
1. Problem-solving in organic chemistry
Studies on the problem-solving performance of students in organic chemistry can be considered as the starting point of a variety of different research efforts and have shaped our understanding in organic chemistry education. The act of problem-solving is inherent to scientific practice and research and is therefore one of the most important goals of teaching and learning science. However, what organic chemists take for granted in their own problem-solving is not comparable to how students solve problems, and it takes them many years to reach a similar state of reasoning (Bodner and Domin, 2000).Four general factors that influence one's problem-solving ability have been examined thoroughly in the literature: problem understanding, strategic knowledge, content and conceptual knowledge, and problem representation (Tsaparlis and Angelopoulos, 2000; Bodner and Herron, 2002; Bodner, 2003). The problems in organic chemistry are classified with regard to their content—mainly as mathematical, non-mathematical, and mechanistic problems. Organic chemistry problem-solving relies more often on judging trends in reactivity, devising mechanisms to predict chemical change, or rationalizing spatial relationships than mathematical calculations. Therefore, solving spectral data and proposing mechanisms or step-by-step synthesis (mechanistic problem-solving) are more frequently used in organic chemistry than in general chemistry and represent a new way of thinking for students enrolled in organic chemistry classes.
In order to determine the important factors of development of expertise in problem-solving, Cartrette and Bodner (2009) compared the problem-solving ability of organic chemistry graduate students and faculty while analyzing spectral data. They observed an interrelation between the individuals' level of content knowledge and the problem representation. Unsuccessful problem-solvers often used single features or isolated facts instead of multiple features presented in the problem statement. Cartrette and Bodner (2009) determined that there is a clear continuum from unsuccessful to successful problem-solvers, even between graduate students and faculty, which was dependent on the experience of graduate students with a particular problem type. They observed additionally that all the successful problem-solvers followed the same algorithmic approach in analyzing spectral data, which is promising for future training. They suggested that problem-solving can be improved and trained by explicitly emphasizing useful and discussing incorrect problem-solving steps that could help students to reflect on incorrect approaches and avoid common errors.
1.1 Mechanistic problem-solving
The use of mechanisms is the flagship of organic chemistry and probably the most challenging part for most students in organic chemistry classes. As the use of mechanisms is inherent to organic chemistry it requires different skills and knowledge than general chemistry, switching from a mostly product-orientated thinking in general chemistry to a more process-orientated thinking, such as designing the step-by-step synthesis and deducing the correct mechanisms.The majority of research investigating mechanistic problem-solving has examined the use of the electron-pushing formalism as a strategy to convey the electron flow or to judge the reactivity and driving force of a mechanistic step. Curved arrows are used as a shorthand notation to convey the electron flow during an organic reaction. When used properly, in accordance with basic physical and chemical concepts, it is not only a powerful tool to explain and predict chemical changes that occur during a reaction but also as a way of making chemical changes explicit. Nevertheless, students at all levels have various problems with the correct application of this problem-solving tool.
In a qualitative study Bhattacharyya and Bodner (2005) investigated the extent to which organic chemistry graduate students make use of the electron pushing formalism while proposing mechanisms for SN1, SN2, and Diels–Alder reactions. They found that even at the graduate level “the curved arrows used in the electron-pushing formalism held no physical meaning for the graduate students” (Bhattacharyya and Bodner, 2005, p. 1405). In their analysis they noticed a gap between students' content or conceptual knowledge and the problem representation. As a result, students have limited understanding of the implicit meaning of the curved-arrow formalism—how and why a reaction is following a certain path. The curved-arrow notion had been used to make the mechanism work or connect different parts of the reaction. Bhattacharyya and Bodner (2005) assumed that some students are unaware that the electron pushing formalism is meant as a tool to explain and predict the stepwise process towards the product. Without an understanding of the properties and meanings that an arrow conveys, the value of using it is lost. The problem-solving strategies shown by the students focused on how to proceed from the starting material to the product, no matter what could be reasonable in a chemical sense, resulting in implausible intermediates or breaking of carbon–carbon bonds. The graduate students in this study setting often started the problem-solving process by matching the atoms and bonds of the starting material and product to identify structural differences between the two. They tended to assume classical reaction types, based on eliminated or added functional groups and let the mechanism work, which Bhattacharyya and Bodner (2005) called the “connect-the-dots” strategy. This study documented that at the graduate level, students were capable of drawing and reproducing mechanistic sequences for common mechanisms, but rarely expressed actual mechanistic problem-solving or the ability to explain the underlying reason for specific steps.
Given these findings it is not surprising that Ferguson and Bodner (2008) found comparable results in a qualitative interview study with chemistry majors enrolled in an organic chemistry class. They used common mechanistic problems, which required the use of arrows. The students in this study used the curved-arrow notation in most of the cases in a correct way while drawing mechanistic steps. However, Ferguson and Bodner (2008) observed a weak connection between the drawing of arrows on the paper level and the underlying concepts or principles that give these arrows a meaning. It looked “real,” but it was in fact not related to a deeper meaning. Students relied on single, unrelated, and erroneous memorized pieces of knowledge and showed little substantial conceptual understanding of the basic concepts (e.g., acid–base chemistry). The inability or unawareness to recall or to apply the appropriate content knowledge was the main barrier that prevented the students from making correct assumptions or proposing reasonable mechanistic steps. Students' deficit in verbally describing the correct mechanistic process and a strong name-dropping behavior was also apparent in the study. Students used terms such as “electrophile,” “reduction,” and “electrophilic addition” as empty envelopes that held no further meaning (Ferguson and Bodner, 2008).
Both studies recognized that there is a remarkable phenomenon emerging from the analysis, namely that students were repeatedly producing the right answer on mechanistic problem-solving tasks without a substantial understanding of the chemistry. Throughout their studies, undergraduate and graduate students seemed to be able to draw correct arrows, but they were not using them as a tool to explain and predict mechanistic steps.
Grove et al. (2012a) investigated the spontaneous use of mechanisms with second-year organic chemistry science majors in a quantitative study, using OrganicPad as the primary data collection tool (Cooper et al., 2009). They noticed that the use of mechanisms placed a high cognitive demand on students and that some students rather opted not to use them. Proposing a mechanism by using the curved-arrows did not seem to be considered useful or helpful to the students when predicting the product in a reaction. The conclusion that a systematic memorization of the common mechanisms seemed to be the usual behavior of students had already been shown in the former studies. However, Grove et al. (2012a) observed that, especially in more complex and unfamiliar tasks that required mechanism use, the students who voluntarily made use of the electron-pushing formalism to solve the problems were more successful. This provides evidence for the fact that students need to experience an actual benefit from using the curved-arrow notation as a problem-solving tool for mechanistic tasks that do not allow us to rely on memorization.
1.2 Future areas of progress in problem-solving
• Make authentic problem-solving strategies explicit• Defining students' successful mechanistic problem-solving strategies
• Emphasize the usefulness and relevance of the curved-arrow notation as a tool to explain and predict mechanistic steps
One important aspect that has been revealed in the studies about problem-solving in organic chemistry is the need for an explicit incorporation of problem-solving strategies in undergraduate and graduate studies. Cartrette and Bodner (2009) concluded that “we should consider teaching problem-solving techniques and strategies that are modeled after those of the “most successful” participants” (Cartrette and Bodner, 2009, p. 657). To mirror the effective strategies used by peer problem-solvers could be one possibility, but we must also consider experts' strategies and their approaches to typical problem-solving scenarios. As shown in Cartrette and Bodner's (2009) study, the successful problem-solving approaches do not greatly differ and may lead to specific training for undergraduate and graduate students.
Bhattacharyya and Bodner (2005) concluded that it is not only important to explicitly address each step and the underlying principles in a mechanism but also to foster awareness of why mechanisms are used in organic chemistry. This emphasis on the relevance of organic synthesis and mechanisms seems to be an important factor to promote meaningful learning (Raker and Towns, 2012a, 2012b). Besides reproducing memorized steps, however, the question of what students are actually doing when successfully solving mechanistic task is still unknown and only tentative models have been proposed based on the former findings (Bhattacharyya, 2014).
With regard to instructional improvement the findings from the studies cited therein indicate the need to develop a variety of diverse exercises and problems that require the critical evaluation of synthetic steps and could help students apply chemical concepts in different problem contexts. These include comparing pKa values of functional groups to evaluate the most likely mechanistic step or reducing the cognitive load by relating and clustering mechanistic steps, e.g., ring opening reactions at bromonium ions and epoxides. Common reaction types, e.g., SN2 or addition reactions are often easily memorized and reproduced, intramolecular reactions and combination of various reaction types in one reaction may initiate a deeper chemical reasoning, because these tasks cannot be easily solved with memorized schemas (Bhattacharyya and Bodner, 2005). As shown in Ferguson and Bodner's study (2008), students seemed to be aware of the inherent nature of organic chemistry, but memorizing every single reaction was still the typical approach. The cognitive effort to use the curved-arrow formalism to derive mechanistic steps was apparently higher than the act of memorizing every presented mechanism.
Future research should establish not only instructional strategies to promote successful reasoning strategies or “chemical questions” that a student should ask while proposing common mechanistic problems but also evaluate which type of tasks actually are useful to improve the mechanistic problem-solving ability. One approach to develop effective learning scenarios for mechanistic problem-solving that has not been considered yet is the learning by errors approach (Ohlsson, 1996). Although it is controversial to discuss how to deal with errors in the classroom, purposefully designed activities to detect and correct students' own performance errors or specifically designed tasks might be valuable, but these await future investigation.
2. Cognitive skills
Organic chemistry is one of the most visual sciences, considering the generation and interpretation of mainly domain-specific symbols and structural representations that have no counterparts in our daily lives. The way molecules are displayed, mechanisms are rationalized, and stereochemical information is presented are all inherent to organic chemistry. The conventions that are used to generate and display structural representations have been developed over a long period of time (Hoffmann and Laszlo, 1991). Lewis structures, stereochemical information, Fischer and Newman projections, and different 2D and 3D representation are just a few examples of how chemists visualize chemical information. Manipulating, translating between, and correctly interpreting these representations are huge challenges to most of the students in a chemistry class (Kozma and Russell, 1997) and require various cognitive skills. The cognitive skills currently under investigation that influence students' performance in organic chemistry classes can be organized into representational competence, spatial ability, and scientific reasoning strategies.2.1 Representational competence
Within the effort to characterize the development of proficiency of students in organic chemistry, the notion of representational competence had been used in the last decade in organic chemistry education to describe the sense-making process of students while engaged in interpreting and transforming different sorts of representations in terms of diagrams, structures or mechanisms.According to Kozma et al. (2000), “Chemists have designed tools and representational systems that mediate between something that they cannot see and something that they can” (Kozma et al., 2000, p. 106). In their narrative analysis, they compared the use of structural representations in laboratory practice, an organic research lab, and a pharmaceutical company to investigate the representational expertise. They concluded that structural representations are central in chemistry and an inherent part of the nature of chemical practice. Kozma and Russell (2005) research furthermore guides the current representational competency research efforts, as they highlighted five levels of representational competence. This extensive research revealed that novice students often did not have the basic knowledge to manage the use of multiple representations during problem-solving, whereas experts were “able to make connections across multiple representations and coordinate the features of these representations to support their discourse about the entities and processes that underlie them all” (Kozma, 2003, p. 213).
Former studies on problem-solving already revealed that deficits in students' ability to translate between structural formula and to understand symbolic representations of molecules influenced their problem-solving behavior; the first step in a problem-solving cycle requires interpreting the information from the given representation to recognize the problem. Bodner and Domin (2000) collected various examples of successful and unsuccessful problem-solvers from all college levels in chemistry with regard to their ability to interpret given organic structures. They found that unsuccessful problem-solvers were unable to translate between verbal-linguistic representations like structural formulas and their respective structural representation. Moreover, a successful problem-solver usually constructed more representations to characterize a given problem, whereas an unsuccessful problem-solver used verbal descriptions. Students in their study tended “to handle chemical formulas and equations that involve these formulas in terms of letters and lines and numbers that cannot correctly be called symbols because they do not represent or symbolize anything that has physical reality” (Bodner and Domin, 2000, p. 27). It is apparent that for those students, there is a gap between the structural representations, which are mainly perceived by surface-level features, such as bonds and atoms, and the physical or chemical meaning that a functional group conveys. One possible reason for this finding has been documented by Ealy and Hermanson (2006) who investigated undergraduate science majors' understanding of various molecular images, ball-stick models, spectroscopic data and the connection between the corresponding chemical concepts, aromaticity, symmetry, and shielding. They noticed that students' understanding of the rules and principles learned in general chemistry—often not covered again in depth in organic chemistry classes—substantially influenced their ability to interpret molecular images. Students in this qualitative study setting struggled to identify aromatic molecules, because they focused on particular atoms and the octet rule and did not consider the delocalization of electrons, a phenomenon inherent to organic chemistry. Yet, they showed a good understanding of electronegativity when explaining shielding effects in a NMR spectrum based on the influence of electronegative atoms (Ealy and Hermanson, 2006). The latter two findings suggest that students have more difficulties to cope with domain-specific organic concepts, such as the electron-delocalization as this construct applies to a group of atoms and exceed the one-atom dimension common in general chemistry; electronegativity generally refers to one type of atom and may thus be easier to apply in organic chemistry. Hence some of the rules and principles learned in general chemistry need to be reconsidered in the organic chemistry context in order to facilitate the interpretation of organic structural representations.
In a qualitative study with faculty and undergraduate science majors enrolled in an organic chemistry class, Domin et al. (2008) followed a comparable research question to the former research study and focused on students' attentional weight when perceiving structural representations. While engaged in categorizing eight α-chloro derivatives that displayed different stereocenters and functionalities on cyclic or acyclic structures, students' categorization behavior revealed their particular choice of relevant cues. Faculty and students in this study mainly chose the displayed functional group as the critical attribute for a grouping, but the basis mentioned for that grouping was different. Students described their grouping mainly as looking for similarities between surface features, whereas the faculty members considered the respective reactivity of a functional group. Faculty and students in an organic chemistry class thus seemed to have a differing perception of structural representations. Our ongoing study (Graulich and Bhattacharyya, 2014) examines the cue selection behavior of science majors enrolled in a first-year organic chemistry class while engaged in categorization tasks. This qualitative study adds additional insight to the former results by using organic chemical reactions. A preliminary analysis indicates that students strongly focused on the surface-level when engaged in categorizing alkene addition reactions.
An additional aspect to the former studies has been given in a study by Strickland et al. (2010). This study investigated the representational competence of organic chemistry graduate students by analyzing the relationship between students' understanding of common organic terms—like acid–base, functional group, or electrophile/nucleophile—and their verbalization of the corresponding structural representations. They described that even at the graduate level students' explanations were often based on very superficial information rather than on process-orientated attributes (e.g. kinetic behavior or thermodynamic parameters). This behavior led them to pay more attention to structural change and limited their interpretation of structural representations. Students could verbally explain the general behavior of electrophiles or nucleophiles, but they had trouble identifying this behavior in the mechanisms presented.
The results of the former studies characterized the performance of undergraduate and graduate students at different points in time and documented in both groups comparable deficits in interpreting structural representation. This raised the question of how representational competence actually develops over time. Grove et al. (2012b) conducted a longitudinal study, which focused on how undergraduate students' use of the curved-arrow notation as a representational formalism changed over a year of instruction. This large-sample study used simple exercises in the predict-the-product format and asked explicitly for drawings of the mechanism. One may suppose that the ability to deal with curved arrows in mechanistic exercises would improve with time and experiences made in class; however, this does not seem to be the case. More than half of the students were not engaged in using the mechanism as an instrument to predict the product. 15–20% of the students went back and included the arrows after having predicted the product. Additionally, the researchers observed that with an increase of proposed mechanistic pathways for the exercises given at the end of the year, the number of erroneous mechanisms increased too. This observation shows that students, even over a certain time of practice, struggle to appropriately apply the curved-arrow notation, which also affects their problem-solving competence as graduate students.
Hand and Choi (2010) analysed multi-modal representations—such as graphs, drawings, and mathematical or chemical equations—that undergraduate students constructed during an organic chemistry laboratory class that used the Science Writing Heuristic approach. They discovered a connection between students' quality of arguments given for an explanation and their use of representations in their lab book. This research reveals that the understanding of a concept or a model can be related to the way the corresponding representations are constructed.
2.2 Spatial reasoning
Beside the broader concept of representational competency, the capability of using visuo-spatial reasoning (among others: Mathewson, 1999; Wu and Shah, 2004; Harle and Towns, 2011; Newcombe and Stieff, 2012) largely influences the performance in organic chemistry (Carter et al., 1987; Pribyl and Bodner, 1987). The representations used in organic chemistry often necessitate the application of strategies to decipher the spatial relationship of structures or diagrammatic representations. These strategies can be analytic or visuo-spatial. For example, determining the stereochemistry of two enantiomers can involve a mental rotation to check the mirror plane or analytical strategies to determine the priority of the substituents, known as the Cahn–Ingold–Prelog R/S designation. Stieff (2007) investigated undergraduate students' and experts' use of both strategies. Experts were much more often engaged in using analytical strategies, whereas students relied on mental rotation of the presented objects and molecules. Stieff (2007) concluded that the use of analytical or rule-based strategies is a result of expertise that allows experts to circumvent mental rotation; a task that becomes more complicated in large molecules with different stereocenters. The analysis showed that visuo-spatial ability seemed to be a prerequisite for success with these particular tasks, along with the flexibility to use alternative strategies during problem-solving and to switch between strategies if mental rotation or where a rule-based strategy could not be applied (Hegarty et al., 2013). The instruction has a direct effect on the choice of the strategy used to manipulate visuo-spatial information. Stieff et al.'s, 2012 research indicates that students often used spatial–imagistic strategies at the beginning of the instruction but increased their use of domain-specific alternative strategies to solve spatial tasks as the class progressed (Stieff et al., 2012). Comparable results have been found in another qualitative study with undergraduate students enrolled in a two semester organic chemistry course (Stieff, 2011). Using think-aloud protocols Stieff (2011) investigated students' use of imagistic and diagrammatic strategies while solving problems with molecular representations, translating between chair and boat conformations and Fischer–Newman projection. These processes are challenging for the students, as one needs to perceive the embedded three-dimensional information given in the representation. The study observed that especially in tasks that required considering the spatial rearrangement of bonds to determine the appropriate reaction path, students rarely focused on the embedded spatial information in a given diagram and often applied a duplication strategy in an attempt to redraw the shape and/or the structure of the given molecules. They overlooked the spatial relationship between the substituents, symbolized by dash-wedge bonds, and produced wrong structures. He concluded that students “appear to manipulate molecular diagrams with heuristics that reify the diagrams instead of recognizing them as representations of the molecular world” (Stieff, 2011, p. 332). However, in translation tasks students tend to rely more often on mental rotation strategies, while increasing substantially the time to solve the given problem.Ferk et al. (2003) determined in a quantitative cross-sectional study that student's perceptions of three-dimensional structures is dependent on the given representation—such as concrete models, photographs, or computer-images—as well as on the complexity of the task (i.e., how many mental processes were necessary in the task—perception, rotation, and reflection). Regardless of the educational level, from primary school to university level, the more processes were incorporated in a task, the more difficult it was for the students. For this reason, he argued for a separate instruction of each mental process.
These studies indicate that not only the act of translating back and forth between structures, but as well the use of different types of structural formula used in the classroom may hinder the learning process. Various structural formulas, skeletal formulas, Newman and Sawhorse projection, and perspective drawings, using dashed and wedged bonds, are usually used inconsistently and without the explicit training.
2.3 Reasoning strategies
Beside the question of how students construct and translate between various types of representations and representational conventions, a host of other studies focused on the nature of students' reasoning skills and strategies that may influence their performance in organic chemistry.Kraft et al. (2010) undertook a qualitative study to understand what kind of cues organic graduate students use for generating meaning while engaged in mechanistic tasks. This report is part of a larger study on representational competence, also described by Strickland et al. (2010). Kraft et al.'s (2010) research focused on the identification of the reasoning strategies that students use. As outlined in this study, proposing mechanistic steps necessitates multi-variate thinking that includes balancing numerous different variables (such as reaction conditions, reactivity of functional groups, or acid–base properties). In their analysis, they found that a majority of the students were using case-base or rule-based reasoning strategies. Only a few were engaged in model-based reasoning, which is considered to be the more successful reasoning strategy, as it provides a transferable internal model of the problem presented. Rule- and case-based reasoning were often triggered by single cue associations, an experience in class, or a memorized rule (i.e., “nucleophiles attack electrophiles” and some molecules are “good leaving groups”). This allowed students in this study to reproduce memorized sequences of steps or mechanisms without a complete understanding. Kraft et al. (2010) discovered that the rules mentioned were often correct but remained factual and were not taken to judge reactivity or to decide between different possible mechanistic steps.
Christian and Talanquer (2012b) studied the use of reasoning strategies that science and engineering majors used in self-initiated study groups in an undergraduate organic chemistry course. They found the same predominant use of rule- and case-based reasoning at the undergraduate level and defined a fourth reasoning mode: symbolic reasoning mode. They used it to classify students' argumentation when they mainly manipulated representations like atoms or bonds on a purely symbolic level without a clear reference to their chemical nature. Students primarily used case- and symbolic reasoning while talking about reactivity and mechanism and spent much more time on static representations instead of discussing process-orientated mechanistic issues (Christian and Talanquer, 2012a). They further argued that the results on the use of reasoning strategies applied by students studying organic chemistry seemed to be a homemade problem. A huge amount of time in class is spent on learning to construct, use and translate structural representations and to visualize structures, which predominantly require rule- and case-based reasoning. Often exams or in-class assessments are organized in a comparable way and influence this narrowed learning focus. This explains why the main cognitive processes students used in their study were basic cognitive processes, such as “remember” and “apply” (as described in Blooms taxonomy) and why they utilized few higher-level cognitive processes involving an evaluative or critical analysis.
A recent qualitative study by De Arellano and Towns (2014) focused on undergraduate science majors' reasoning behaviors investigating their argumentation when asked to predict products and mechanisms for alkyl halide reactions. The researchers used Toulmin's model of argumentation to identify students' source and quality of reasoning. Those students who were successful showed an appropriate connection between the property of a reagent, nucleophile, or base and the corresponding reaction type, SN1, or elimination reaction as well as knowledge about intermediate and mechanistic steps of the reactions. However, they also found that many students seemed to be able to produce the right product even without a substantial understanding. These results provide further evidence that a constant focus on correctly using and applying the basic chemical concept in organic chemistry is crucial to improve the organic chemistry classroom practice. Furthermore students, who try to rationalize mechanisms instead of reproducing them, should be valued for their effort.
2.4 Future areas of progress to improve students' cognitive skills
• Determine how structural cues influence students' use of analytical and diagrammatic strategies• Engage students in model-based reasoning
• Analyze how to diversify students' reasoning strategies
The findings from research on general cognitive skills imply that students should constantly be engaged in the use of strategies to deal appropriately with structural representations in organic chemistry. The act of interpreting common representations should become a large part of the discourse in class, as this is the basis for judging properties or reactivities as well as part of successfully approaching problems. Various educational technologies are now available for multi-representational visualization of objects or phenomena, via animations, simulations and others. These computer-assisted environments can assist students in understanding various representations (among others: Kozma and Russell, 1997; Wu and Shah, 2004; Stieff and Wilensky, 2003).
Stieff et al. (2012) showed that the use of domain-specific strategies increased with instruction. Thus, additional time should be spent in class on the application of these strategies, such as how to visualize molecular structures and how to translate between different molecular representations (Stieff, 2011). With regard to the usefulness of using the textbook to teach the translation between Fischer and Newman projection formulae, Kumi et al. (2013) analyzed textbooks' strategy suggestions on how to transfer from Fischer to Newman projection and vice versa. She found that only a few textbooks gave a thorough stepwise approach of how to rotate the molecule and translate between the different projections. Therefore, instructors should be aware that some textbooks may not be suitable for presenting the different molecular perspectives and strategies used to translate between them.
There is often no consistent agreement on how and when to use the variety of conventions to visualize an organic molecule in the classroom, e.g., perspective drawings, dashed-wedged bonds or condensed structural formulae. Systematic investigations are needed to determine how this practice affects the development of representational competence and if certain structural cues present barriers for students' understanding and problem-solving ability.
While relying heavily on structural or diagrammatic representation in teaching the corresponding transfer from a representation to its verbal description and vice versa, the adequate use of the corresponding verbs to express reactivity and properties has taken a back seat. Future research should address how the use of chemical language affects learning and understanding in chemistry and whether emphasizing the connection between a structure and its underlying meaning by verbalizing the properties supports a deeper understanding.
Kraft et al. (2010) further proposed that organic chemistry has to be presented as a multi-variate system or what Ribeiro and Pereira (2012) called a “constitute pluralism.” Reasoning in organic chemistry involves various cognitive processes at the same time, such as balancing influential factors on a reaction step, relating concepts to structural representation and keeping track of the electron flow with the curved arrow notation. According to Kuhn et al. (2009) and Hmelo-Silver et al. (2007), this type of cognitive processing needs explicit instruction and specific reasoning strategies. Kraft et al. (2010) suggested that instructors should consider model-building activities that could help students to build up the important model-based reasoning skills missing in students' reasoning, as well as giving direct feedback on devising mechanistic steps and the use of arrows. The predominant use of rule- and case-based reasoning suggests that students risk a cognitive overload while engaged in mechanistic problem-solving. Strickland et al. (2010) claimed that one reason for the problems encountered could be the missing emphasis on metacognition, i.e., students are not spending enough time in class on the critical analysis of their own constructed structural representations. Future research is needed to determine how to initiate successful reasoning modes and to encourage students to reflect on their own reasoning and decision-making processes. De Arellano and Towns (2014) further suggest making “reagent property–reaction type relationships” explicit in instruction, while also providing diagnostic tools that give instructors valuable ways to determine students' conceptions or reasoning resources.
One aspect that became evident in the studies on reasoning strategies is that students tend to be very minimalistic when learning and studying (Christian and Talanquer, 2012b). This is particularly the case for the transition from product-orientated general chemistry thinking towards process-orientated reasoning about mechanisms (Grove et al., 2012b). When students are not experiencing the value of being engaged in higher-order thinking skills or model-based reasoning, it is not likely that they adopt cognitively more demanding reasoning modes. This aspect indicates the necessity for a critical analysis of the current teaching and assessment practice that mostly require the recall of memorized facts. A closer collaboration between research in organic chemistry education and its practice would be beneficial to diversify and improve future teaching and learning.
3. The nature of students' conceptual knowledge
A solid content knowledge is a prerequisite to construct and interpret structures in a meaningful way. Hence several studies tried to capture students' alternative conceptions or cognitive organization of knowledge. Nash et al. (2000) conducted a small quantitative study to uncover the interrelation of freshman chemistry majors' conceptual knowledge. They found that over a semester the knowledge structure organization, displayed with an ordered-tree technique, increased, and common organic terms were more hierarchically organized—which could also be related to their performance in class. The chunking of concepts relied more on surface similarity than on conceptual similarity but became more conceptual over the period of instruction. Building a more conceptual organization as seen in experts' knowledge structure seemed to be an indicator for successful learning. However, the students' understanding of the chemical terms used in the concept maps had not been determined in their study. As such the question how a correct definition of a chemical term given by a student relates to the actual application in a problem-solving context is still missing.Taagepera and Noori (2000) and Taagepera et al. (2002) used the knowledge space theory to describe the knowledge structure of undergraduate biology majors enrolled in an organic chemistry class. In this quantitative study they also observed an increase in students' cognitive organization of the knowledge over a year of instruction; however, the interconnection of concepts was persistently weak. Students still had various alternative conceptions about common chemical concepts, reaction types, bond polarity or bonding, and used various algorithms. Their understanding of bonding in organic chemistry appeared to be very superficial, as students did not seem to differentiate between hydrogens bonded to carbon or to oxygen, which makes the understanding of acid–base chemistry or hydrogen bonding difficult. This study clearly shows that the situation for non-majors in an organic chemistry classroom might be much more challenging compared to their peers in chemistry.
Rushton et al. (2008) used the ACS exam for organic chemistry to investigate the alternative conceptions that senior chemistry students have before graduating in chemistry. In this qualitative study design, they observed that senior students demonstrate various aspects of model confusion, mainly about the correct application of concepts and understanding of organic terms and their meanings. The source of alternative misconceptions appears to be very fragmented. Students evaluated the stability of a product instead of the feasibility of a mechanism or misapplied the term “aromaticity” to hyperconjugated molecules. Rushton et al. (2008) observed that judging dynamic processes compared to static images, for instance the preferred position on a cyclohexane ring or a prediction of the preferred product in a SN1, is more difficult for students. These observations are consistent with the research findings on students' problem-solving behavior and show increasing deficits in students' understanding going from chemistry majors to non-majors. Investigations on how the conceptual knowledge structure of chemistry students' develops at the graduate level and what non-majors actually remember after several years of study would complete the bigger picture.
3.1 Structure–property relationships
One of the biggest ideas in chemistry is the concept of structure–property relationships. This seems to lie at the heart of successful performance in organic chemistry, expressing chemical meaning through structural representations and interpreting the meaning of those. The use and understanding of Lewis structures had been examined thoroughly by Cooper et al. (2010). They conducted a large mixed-method study with undergraduates enrolled in a general and organic chemistry class, to determine their difficulties in drawing various Lewis structures. In their analysis, they observed that general and organic chemistry students' performance in constructing the right Lewis structures was comparable and dependent on how the structural formula was presented. The students' difficulties increased with the complexity of the molecule. Very few students could explain the purpose of a Lewis structure—namely to infer chemical information, molecular shape, and the influence of intermolecular forces from it. These results may originate from dominant principles learned in general chemistry classes, such as the octet rules, or a poorly understood use of Lewis structures as “shorthand” to convey shape and properties. Cooper et al. (2013) further determined if and how students were using molecular representations to make predictions of properties. They described that, aside from the fragmented conceptual knowledge of students and the misapplication of instructional rules of thumb (i.e., the octet rule and “like dissolve like”), individual assumptions and heuristics strongly influenced their perception.Henderleiter et al. (2001) found comparable results in a study with undergraduate science majors enrolled in an organic chemistry course. They investigated the students' understanding of the hydrogen-bonding concept after completing the second year of organic chemistry. Their results showed that students still held some alternative conceptions, which prevented the successful determination of boiling point differences or effects in NMR and IR spectroscopy as well as explanation of the influence on the outcome of organic reactions. As described in the presented studies, basic principles learned in general chemistry are often not discussed again in the organic chemistry classroom and the additional content knowledge, e.g., the notion of steric hindrance, seems to impede students' understanding of the basic chemical concepts.
3.2 Acid–base concepts
The acid–base concept is ubiquitously used in organic chemistry when rationalizing mechanisms, determining the influence of reaction conditions, and devising synthetic steps. Recognizing acidity and comparing compounds necessitate a robust understanding of acidic strength and the application of the acid–base concepts in use, Brønsted–Lowry and Lewis theory. Bhattacharyya (2006) undertook a qualitative study with graduate students to determine the nature of their mental models when applying different acid–base concepts. The most often stated characteristic to describe acidity was “bond strength,” whereas steric or solvent effects were less often mentioned. So far “their models had a descriptive quality without much predictive capability” (Bhattacharyya, 2006, p. 244). Their explanations were often based on one recalled characteristic but did not consider the interplay of multiple aspects of acid–base theory.Some studies have taken a closer look to define what kind of intuitive thinking (Evans, 2003) students use to determine acid–base properties and how this influences the quality of their decision-making process. McClary and Talanquer (2011a, 2011b) described various heuristic strategies in their study with undergraduate organic chemistry students while engaged in ranking acids, especially organic molecules. The participants in this qualitative study were science and chemistry majors enrolled in their first-year organic chemistry course. They noticed various heuristics that allowed students to eliminate cues and to focus on one single attribute, for instance the presence of a functional group that is considered to be an acid. However, McClary and Talanquer (2011b) stated that the students were successful over 40% of the time by using heuristic strategies but that less than 8% based their explanation on acceptable scientific concepts related to acid–base chemistry. The use of heuristic strategies seems to be task-dependent and may be triggered by the selection of given tasks (McClary and Talanquer, 2011a). Nevertheless students tend to develop individual mental models of acids and acidity based on various ideas and intuitive assumptions of the behaviour of acids. In a follow-up quantitative research study, McClary and Bretz (2012) developed an assessment tool for alternative conceptions held by science majors on acid strength. In summarizing their findings they noted that students mainly held two alternative conceptions namely: “functional group determines acid strength” and “stability determines acid strength.” Both conceptions are primarily based on structure related features and less on underlying properties.
Cartrette and Mayo (2011) carried out a qualitative study with organic chemistry majors and investigated how students solved organic problem-solving exercises that required the application of acid–base theory. They reported that the students' declarative knowledge was mainly correct and that the students primarily referred to Brønsted–Lowry theory when explaining the terms of acid–base behaviour. Cartrette and Mayo (2011) declared that although organic chemistry majors students were able to compare the acidity of organic molecules in terms of resonance, inductive effects and electronegativity, they struggled to apply the concepts while doing their problem-solving exercises. Cartrette and Mayo (2011) assumed that a poor understanding of the Lewis acid concept prevented them from drawing solid connections between acid–base concepts and the terms electrophile and nucleophile. These results document that the undergraduate students' understanding on acid–base chemistry is frequently dominated by intuitive assumptions on acid properties. Organic chemistry majors had a more diversified conception, but struggle to apply these concepts in problem-solving contexts.
3.3 Future areas of progress
• Reinforce the interpretation of structure–property relationships in organic chemistry• Investigation of students' use of shortcut reasoning strategies with regard to the basic concepts
• Emphasize the application of chemical concepts in various contexts
Studies on the nature of students' content knowledge in organic chemistry exposed a picture of very scattered knowledge and diffused mental models that resulted in miscellaneous intuitive assumptions about structures and structure–property relationships. A complete picture of how conceptual knowledge evolves in organic chemistry and how the pieces of knowledge become interconnected over time is hard to grasp, as there seems to be a gap between a reproducible definition of chemical concepts and its actual application. It has been shown that students are mostly algorithmic thinkers and struggle to construct a solid conceptual knowledge that could help them to integrate new learned knowledge in a sustainable manner. The reliance on intuitive strategies and heuristics strongly guides their reasoning process and is a central resource in the students' decision-making process. Heuristic shortcut strategies help students generate an idea while judging the reactivity of a chemical process, but they also can, in the absence of the required knowledge base, lead to misconceptions and the recall of wrong associations. Future research should provide additional explanatory frameworks for the sources of intuitive assumptions held by students, compared to the recent research efforts on heuristics in general chemistry (Cooper et al., 2013; Maeyer and Talanquer, 2013; Becker and Cooper, 2014). Additional studies are necessary to determine how students can build up successful heuristics and use effective domain-specific heuristics (Graulich et al., 2012). A thorough understanding of heuristic thinking throughout all chemistry disciplines would help inform appropriate learning scenarios and assessments.
The aspect of metacognition has frequently been mentioned in the above studies, because the students were lacking the understanding of when and how to apply a specific chemical concept in a problem-solving context. McClary and Talanquer summarize that “the challenge seems to be in helping students better recognize their use of heuristics, when to apply them, and how to monitor and exert control over their application” (McClary and Talanquer, 2011b, p. 1451).
4. Epistemological development
Some research studies focused on the overall experience in learning and becoming a practitioner in organic chemistry. Anderson and Bodner's (2008) study can be considered as the first dedicated to examining an overall course experience in an organic chemistry class. In a case study about a student named Parker, they exemplified the experience of many students who had been successful in general chemistry but struggled in organic chemistry. Anderson and Bodner (2008) gave an overview of the emergent problems during an organic class, e.g., the ability to handle structural representations, the appropriate use of the curved-arrow notation and the process-related thinking about mechanisms. They described the reasoning modes expressed by the students in their study as instrumental or relational learners as the difference between students who were able to see the patterns and used the chemical concepts for their reasoning and those who were following memorized rules that did not allow transfer. Although the use of mechanisms had been explicitly expressed in the classroom under investigation, the students seemed not to value their use. Those findings are consistent with the results reported in detail in other studies and are viewed from a more holistic perspective in this study.Other research efforts considered the personal development in organic chemistry going from undergraduate and graduate students to practicing chemists. Bhattacharyya (2008) outlined the epistemic development from students to experienced chemists, with regard to their conceptual knowledge development and their organic synthesis problem-solving skills (Bhattacharyya and Bodner, 2014). The researchers presented various steps in the progression from student to practitioner and concluded that a deep level of conceptual understanding was only reached at a high level of expertise, mainly after graduate school. Before that point, students constructed different types of knowledge along their way, such as learning the terminology and adopting the use of heuristics for complex mental models of chemical concepts. The main difference between students and experienced chemists was that at a later stage, the conceptualization of knowledge occurred based on the chemical processes and phenomena at the molecular level and chemical concepts have been used as tools with predictive value. This important mindset did not seem to develop until leaving graduate school. This is reflected by the other research studies in organic chemistry education reported herein, which documented comparable deficits and a consistently weak performance at the undergraduate and graduate level.
Grove and Bretz (2010) investigated the epistemological development during an overall class experience of undergraduate students taught in a spiral curriculum (Grove et al., 2008). This qualitative study analyzed students' perception and expectation of organic chemistry. They found that students especially evoked an idea of “straightforwardness”. In general chemistry the course content seemed to be perceived as dualistic; straightforward between problem and answer, whereas in organic chemistry a relativistic perceptive needed to be adopted, evaluating various influences, i.e., balancing the variables between elimination or substitution. Therefore, this dualistic perception could constitute a barrier for understanding the mindset in organic chemistry (Grove and Bretz, 2010). Within this study Grove and Bretz (2012) also examined how the meaningful learning develops in an organic chemistry class. They stated that students strongly rely on rote-memorization and that the most important factor that hindered meaningful learning was the perceived lack of relevancy of the class itself.
Another recent quantitative study focusing on the overall performance of undergraduate students in organic chemistry was conducted by Szu et al. (2011). They compared several factors, such as overall course grades, performance on concept maps and problem-solving tasks, and their relation to students' performance in the class. Positive correlations were found between the final course grade and a high prior GPA or the habit of a weekly studying frequency since the beginning of the class. It is not surprising that procrastination seemed to be a detriment to success. Szu et al. (2011) confirmed that a high level of conceptual understanding as well as an understanding of how course concepts are interrelated was an indicator for success in organic chemistry.
4.1 Future area of progress
• Make organic chemistry practices relevant for students through authentic practice• Accentuate the relativistic mindset in organic chemistry as compared to general chemistry
• Foster metacognitive and learning strategies
As outlined by Bhattacharyya (2014), the epistemic development of organic chemistry students can be promoted by emphasizing their identity formation by including authentic practice that may help them relate meaning to learned declarative and procedural knowledge. The “realness” of problems used in the classroom can be addressed by including more open-ended and authentic synthesis problems, as well as a regular cycle of feedback and revision to promote ownership development. Grove and Bretz (2012) also recognized the importance of the relevancy of taught material as a main initiator for meaningful learning in organic chemistry and encouraged the emphasis of the relativistic mindset in organic chemistry through the inclusion of multistep-synthesis, or competing reaction paths. It became apparent that there is a difference between the nature of organic chemistry and the students' perception of it. Anderson and Bodner (2008) also stated that the students poorly understood the function of organic mechanisms and that students need to make the explicit experience how a mechanism is actually helpful in predicting outcomes and balancing competing mechanistic paths.
Where are we going from here?
Previous research studies have given us a broad view of the nature of students' understanding and the various factors that influence undergraduate and graduate students' success in organic chemistry.The implications for future instruction and research outlined in the research reports reviewed can be condensed to one decisive aspect that researchers and instructors must address in the future, mainly to illustrate the why and how of organic chemistry. In all domains of organic chemistry—analyzing data, solving problems, proposing mechanisms, and interpreting structures and diagrams—making the implicit explicit by explaining how and why a chemical concept or an inherent convention is applied. Looking at the reported research results, one gets the impression that with the current practice, students at all levels obviously do what we want them to do without knowing what we want them to know. This is a problematic situation, as alternative conceptions, missing chemical knowledge, or erroneous reasoning strategies seem to be hidden under an apparently correct answer. Students struggle to apply their declarative knowledge in actual problem-solving contexts. Future research needs to further uncover the multiple factors that led to this barrier.
One reason for this overall finding is that teaching organic chemistry usually resembles a rather descriptive collection of seemingly unrelated reactions, than actively rationalizing mechanistic steps. We need to establish how to promote meaningful and sustainable learning and reasoning in organic chemistry. One decisive aspect may be to determine what actually comprises “mechanistic reasoning.” What are organic chemistry experts doing while solving mechanisms and how can we translate this into an effective teaching practice? How do students develop expertise in mechanistic reasoning?
Moreover, as shown in various research studies, the rules and principles learned in the general chemistry class are often not translated appropriately into the organic chemistry classroom, although they are often taken for granted. Many domain-specific organic concepts, such as hyperconjugation, aromaticity, and resonance are not straightforward for the students, as the mindset in general chemistry is rather focusing on single-atoms or small entities. Hence future research initiatives should aim at clarifying what knowledge pieces students are actually translating from general chemistry into organic chemistry and how erroneous individual assumptions can be addressed in the organic chemistry classroom.
The urgent demand for research-based instructional strategies also reflects the need to think differently about how we assess chemical understanding. It is evident from the current studies that students still rely heavily on rote-memorization and that traditional give-the-product exercises are frequently solved without a deeper understanding. A combination of appropriate instructional strategies and the corresponding assessment is compulsory to change students' perception and their learning behavior in the long run.
Although the majority of research reports described the deficiencies of students' understanding rather than their actual resources, it helped to establish research-based evidence that can guide future teaching initiatives. Currently, we have a good notion about the obstacles and a good selection of common errors, misconceptions, and faulty strategies in organic chemistry. To fully capture and address students' understanding in organic chemistry, research efforts in organic chemistry slowly shift their focus towards a more positive description of students' resources and learning progressions that allow us to establish a bigger and more complete picture of students' understanding and tailor effective instructional designs. Emergent research areas that have marginally been addressed in organic chemistry education research include students' motivations and beliefs about organic chemistry as well as their use of metacognitive strategies. Moreover, longitudinal or cross-sectional studies are needed to describe the expertise development from undergraduate to graduate students, especially with regard to the fostering of crosscutting concepts (e.g. the concept of energy or acid–base theory), and research initiatives that translate this research into practice.
Research in organic chemistry education has now passed the point of anecdotal experiences and revealed to offer a large variety of discipline-based research initiatives. Nevertheless it became apparent that research in organic chemistry education is still a patchwork quilt that necessitates more guided research efforts and a clear use of terms to build a well-defined research portfolio.
Much has been done to uncover the nature of students' understanding, but a huge part still remains hidden.
References
- Anderson T. L. and Bodner G. M., (2008), What can we do about ‘Parker’? A case study of a good student who didn't ‘get’ organic chemistry, Chem. Educ. Res. Pract., 9, 93–101.
- Becker N. M. and Cooper M. M., (2014), College chemistry students' understanding of potential energy in the context of atomic–molecular interactions, J. Res. Sci. Teach., 51, 789–808.
- Bhattacharyya G., (2006), Practitioner development in organic chemistry: how graduate students conceptualize organic acids, Chem. Educ. Res. Pract., 7, 240–247.
- Bhattacharyya G., (2008), Who am I? What am I doing here? Professional identity and the epistemic development of organic chemists, Chem. Educ. Res. Pract., 9, 84–92.
- Bhattacharyya G., (2014), Trials and tribulations: student approaches and difficulties with proposing mechanisms using the electron-pushing formalism, Chem. Educ. Res. Pract., 15, 594–609.
- Bhattacharyya G. and Bodner G. M., (2005), “It Gets Me to the Product”: How Students Propose Organic Mechanisms, J. Chem. Educ., 82, 1402–1407.
- Bhattacharyya G. and Bodner G. M., (2014), Culturing reality: how organic chemistry graduate students develop into practitioners, J. Res. Sci. Teach., 51, 694–713.
- Bodner G. M., (2003), Problem-solving: the difference between what we do and what we tell our students to do, Univ. Chem. Educ., 7, 1–9.
- Bodner G. M. and Domin D. S., (2000), Mental models: the role of representations in problem-solving in chemistry, Univ. Chem. Educ., 4, 24–30.
- Bodner G. M. and Herron J. D., (2002), Problem-solving in chemistry, in Gilbert J. K., De Jong O., Justi R., Treagust D. F. and Van Driel J. H., (ed.), Chemical education: towards research-based practise, Dordrecht: Kluwer, pp. 235–266.
- Carter C. S., Larussa M. A. and Bodner G. M., (1987), A study of two measures of spatial ability as predictors of success in different levels of general chemistry, J. Res. Sci. Teach., 24, 645–657.
- Cartrette D. P. and Bodner G. M., (2009), Non-mathematical problem solving in organic chemistry, J. Res. Sci. Teach., 47, 643–660.
- Cartrette D. P. and Mayo P. M., (2011), Students' understanding of acids/bases in organic chemistry contexts, Chem. Educ. Res. Pract., 12, 29–39.
- Christian K. and Talanquer V., (2012a), Content-Related Interactions in Self-initiated Study Groups, Int. J. Sci. Educ., 34, 2231–2255.
- Christian K. and Talanquer V., (2012b), Modes of reasoning in self-initiated study groups in chemistry, Chem. Educ. Res. Pract., 13, 286–295.
- Cooper M. M., Corley L. M. and Underwood S. M., (2013), An investigation of college chemistry students' understanding of structure–property relationships, J. Res. Sci. Teach., 50, 699–721.
- Cooper M. M., Grove N. P., Pargas R., Bryfczynski S. P. and Gatlin T., (2009), OrganicPad: an interactive freehand drawing application for drawing Lewis structures and the development of skills in organic chemistry, Chem. Educ. Res. Pract., 10, 296–301.
- Cooper M. M., Grove N., Underwood S. M. and Klymkowsky M. W., (2010), Lost in Lewis structures: an investigation of student difficulties in developing representational competence, J. Chem. Educ., 87, 869–874.
- De Arellano D. C.-R. and Towns M., (2014), Students understanding of alkyl halide reactions in undergraduate organic chemistry, Chem. Educ. Res. Pract., 15, 501–515.
- Domin D. S., Al-Masum M. and Mensah J., (2008), Students' categorizations of organic compounds, Chem. Educ. Res. Pract., 9, 114–121.
- Ealy J. B. and Hermanson J., (2006), Molecular images in organic chemistry: assessment of understanding in aromaticity, symmetry, spectroscopy, and shielding, J. Sci. Educ. Technol., 15, 59–68.
- Evans J. S. B., (2003), In two minds: dual-process accounts of reasoning, Trends Cognit. Sci., 7, 454–459.
- Ferguson R. and Bodner G. M., (2008), Making sense of the arrow pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9, 102–113.
- Ferk V., Vrtacnik M., Blejec A. and Gril A., (2003), Students' understanding of molecular structure representations, Int. J. Sci. Educ., 25, 1227–1245.
- Goodwin W. M., (2008), Structural formulas and explanation in organic chemistry, Found. Chem., 10, 117–127.
- Graulich, N. and Bhattacharyya G., (2014), manuscript in preparation.
- Graulich N., Tiemann R. and Schreiner P. R., (2012), Heuristic chemistry-a qualitative study on teaching domain-specific strategies for the six-electron case, Chem. Educ. Res. Pract., 13, 337–347.
- Grove N. P. and Bretz S. L., (2010), Perrys scheme of intellectual and epistemological development as a framework for describing student difficulties in learning organic chemistry, Chem. Educ. Res. Pract., 11, 207–211.
- Grove N. P. and Bretz S. L., (2012), A continuum of learning: from rote memorization to meaningful learning in organic chemistry, Chem. Educ. Res. Pract., 13, 201–208.
- Grove N. P., Cooper M. M. and Cox E. L., (2012a), Does Mechanistic Thinking Improve Student Success in Organic Chemistry? J. Chem. Educ., 89, 850–853.
- Grove N. P., Cooper M. M. and Rush K. M., (2012b), Decorating with Arrows: Toward the Development of Representational Competence in Organic Chemistry, J. Chem. Educ., 89, 844–849.
- Grove N. P., Hershberger J. W. and Bretz S. L., (2008), Impact of a spiral organic curriculum on student attrition and learning, Chem. Educ. Res. Pract., 9, 157–162.
- Hand B. and Choi A., (2010), Examining the impact of student use of multiple modal representations in constructing arguments in organic chemistry laboratory classes, Res. Sci. Educ., 40, 29–44.
- Harle M. and Towns M., (2011), A Review of Spatial Ability Literature, Its Connection to Chemistry, and Implications for Instruction, J. Chem. Educ., 88, 351–360.
- Hegarty M., Stieff M. and Dixon B. L., (2013), Cognitive change in mental models with experience in the domain of organic chemistry, J. Cognit. Psychol., 25, 220–228.
- Henderleiter J., Smart R., Anderson J. and Elian O., (2001), How do organic chemistry students understand and apply hydrogen bonding? J. Chem. Educ., 78, 1126–1130.
- Hmelo-Silver C. E., Marathe S. and Liu L., (2007), Fish swim, rocks sit, and lungs breathe: expert-novice understanding of complex systems, J. Learn. Sci., 16, 307–331.
- Hoffmann R. and Laszlo P., (1991), Representation in Chemistry, Angew. Chem., Int. Ed. Engl., 30, 1–16.
- Kozma R., (2003), The material features of multiple representations and their cognitive and social affordances for science understanding, Learn. Instr., 13, 205–226.
- Kozma R. B. and Russell J., (1997), Multimedia and understanding: expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 34, 949–968.
- Kozma R. and Russell J., (2005), Students Becoming Chemists: Developing Representational Competence, Visualization in Science Education, Springer, pp. 121–145.
- Kozma R., Chin E., Russell J. and Marx N., (2000), The roles of representations and tools in the chemistry laboratory and their implications for chemistry learning, J. Learn. Sci., 9, 105–143.
- Kraft A., Strickland A. M. and Bhattacharyya G., (2010), Reasonable reasoning: multi-variate problem-solving in organic chemistry, Chem. Educ. Res. Pract., 11, 281–292.
- Kuhn D., Pease M. and Wirkala C., (2009), Coordinating the effects of multiple variables: a skill fundamental to scientific thinking, J. Exp. Child Psychol., 103, 268–284.
- Kumi B. C., Olimpo J. T., Bartlett F. and Dixon B. L., (2013), Evaluating the effectiveness of organic chemistry textbooks in promoting representational fluency and understanding of 2D–3D diagrammatic relationships, Chem. Educ. Res. Pract., 14, 177–187.
- Maeyer J. and Talanquer V., (2013), Making predictions about chemical reactivity: assumptions and heuristics, J. Res. Sci. Teach., 50, 748–767.
- Mathewson J. H., (1999), Visual-spatial thinking: an aspect of science overlooked by educators, Sci. Educ., 83, 33–54.
- McClary L. M. and Bretz S. L., (2012), Development and Assessment of A Diagnostic Tool to Identify Organic Chemistry Students' Alternative Conceptions Related to Acid Strength, Int. J. Sci. Educ., 34, 2317–2341.
- McClary L. M. and Talanquer V., (2011a), College chemistry students' mental models of acids and acid strength, J. Res. Sci. Teach., 48, 396–413.
- McClary L. M. and Talanquer V., (2011b), Heuristic Reasoning in Chemistry: Making Decisions About Acid Strength, Int. J. Sci. Educ., 33, 1433–1454.
- Nash J. G., Liotta L. J. and Bravaco R. J., (2000), Measuring conceptual change in organic chemistry, J. Chem. Educ., 77, 333–337.
- Newcombe N. S. and Stieff M., (2012), Six Myths About Spatial Thinking, Int. J. Sci. Educ., 34, 955–971.
- Ohlsson S., (1996), Learning from performance errors, Psychol. Rev., 103, 241.
- Pribyl J. R. and Bodner G. M., (1987), Spatial ability and its role in organic chemistry: a study of four organic courses, J. Res. Sci. Teach., 24, 229–240.
- Raker J. R. and Towns M. H., (2012a), Designing undergraduate-level organic chemistry instructional problems: seven ideas from a problem-solving study of practicing synthetic organic chemists, Chem. Educ. Res. Pract., 13, 277–285.
- Raker J. R. and Towns M. H., (2012b), Problem types in synthetic organic chemistry research: implications for the development of curricular problems for second-year level organic chemistry instruction, Chem. Educ. Res. Pract., 13, 179–185.
- Ribeiro M. A. P. and Pereira D. C., (2012), Constitutive Pluralism of Chemistry: Thought Planning, Curriculum, Epistemological and Didactic Orientations, Sci. Educ., 22, 1809–1837.
- Rushton G. T., Hardy R. C., Gwaltney K. P. and Lewis S. E., (2008), Alternative conceptions of organic chemistry topics among fourth year chemistry students, Chem. Educ. Res. Pract., 9, 122–130.
- Stieff M., (2007), Mental rotation and diagrammatic reasoning in science, Learn. Instr., 17, 219–234.
- Stieff M., (2011), When is a molecule three dimensional? A task-specific role for imagistic reasoning in advanced chemistry, Sci. Educ., 95, 310–336.
- Stieff M., Ryu M., Dixon B. and Hegarty M., (2012), The Role of Spatial Ability and Strategy Preference for Spatial Problem Solving in Organic Chemistry, J. Chem. Educ., 89, 854–859.
- Stieff M. and Wilensky U., (2003), Connected Chemistry – Incorporating Interactive Simulations into the Chemistry Classroom, J. Sci. Educ. Tech., 12, 285–302.
- Strickland A. M., Kraft A. and Bhattacharyya G., (2010), What happens when representations fail to represent? Graduate students' mental models of organic chemistry diagrams, Chem. Educ. Res. Pract., 11, 293–301.
- Szu E., Nandagopal K., Shavelson R. J., Lopez E. J., Penn J. H., Scharberg M. and Hill G. W., (2011), Understanding Academic Performance in Organic Chemistry, J. Chem. Educ., 88, 1238–1242.
- Taagepera M. and Noori S., (2000), Mapping Students' Thinking Pattern in Learning Organic Chemistry by the Use of Knowledge Space Theory, J. Chem. Educ., 77, 1224–1229.
- Taagepera M., Arasasingham R. D., Potter F., Soroudi A. and Lam G., (2002), Following the Development of the Bonding Concept Using Knowledge Space Theory, J. Chem. Educ., 79, 756–762.
- Tsaparlis G. and Angelopoulos V., (2000), A model of problem solving: its operation, validity, and usefulness in the case of organic-synthesis problems, Sci. Educ., 84, 131–153.
- Wu H.-K. and Shah P., (2004), Exploring visuospatial thinking in chemistry learning, Sci. Educ., 88, 465–492.
| This journal is © The Royal Society of Chemistry 2015 |