One-pot synthesis of fluorescent and cross-linked polyphosphazene nanoparticles for highly sensitive and selective detection of dopamine in body fluids†
Abstract
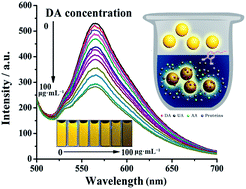
Highly cross-linked and monodisperse polyphosphazene (PZS) nanoparticles exhibiting strong fluorescence were prepared by the facile one-pot polycondensation of hexachlorocyclotriphosphazene and 4′,5′-dibromofluorescein (DBF). Fluorescent DBF units were ‘immobilized’ and ‘isolated’ in the cross-linked structures to effectively overcome their concentration-quenching effect and improve their photobleaching properties. The resulting DBF–PZS nanoparticles emitted bright yellow fluorescence at all concentrations and exhibited excellent resistance to photobleaching as well as interference from bio-molecules such as proteins, ascorbic acid and uric acid. Intriguingly, the fluorescence of the DBF–PZS nanoparticles was linearly quenched by DA in the range of 0.5 to 15 μg mL−1 DA concentration via photoinduced charge transfer. Therefore, the use of DBF–PZS nanoparticles represents a simple but effective, highly sensitive and selective detection method with a direct read out for DA in biological fluids.

 Please wait while we load your content...
Please wait while we load your content...