Novel exocyclic enamides: synthesis and evaluation of the fungicidal activities of 5-methylene-2-(trifluoromethyl)morpholin-3-one derivatives†
Abstract
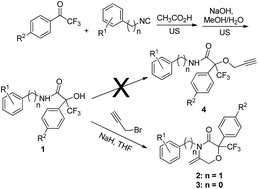
Exocyclic enamides have been used extensively in the synthesis of natural products and biologically active substances. A facile and efficient protocol has been developed for the synthesis of a series of new 5-methylenemorpholin-3-one derivatives, which are exocyclic enamides, based on the reactions of trifluoroatrolactamides with propargyl bromide in the presence of sodium hydride. The biological evaluation of these compounds showed that some of them exhibited very good in vitro fungicidal activity against P. oryzae. For example, compounds 2d and 2e gave fungicidal activities of 50% at a concentration of 0.9 μg mL−1, whilst the control compound fenaminstrobin gave 80% activity at the same concentration.

 Please wait while we load your content...
Please wait while we load your content...