New hybrid materials based on halogenated metalloporphyrins for enhanced visible light photocatalysis
Abstract
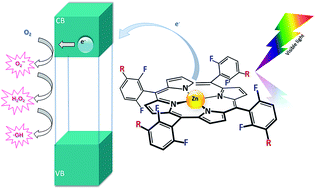
Photophysical and photochemical studies on 5,10,15,20-tetrakis(2,6-difluoro-5-N-methylsulfamylophenyl)porphyrin (F2PMet) and its cobalt(III) and zinc(II) complexes, including spectroscopic characteristics, photostability and photocatalytic activity, were carried out. The hybrid materials resulting from adsorption of these tetrapyrroles at the surface of titanium dioxide were prepared and examined in terms of their morphological, optical and functional properties applying absorption spectroscopy, scanning electron microscopy (SEM), photoelectrochemistry and photocatalytic tests. Our studies revealed that MF2PMet@TiO2 photocatalysts can be considered as the hybrid organic/inorganic photoactive materials enabling photodegradation of a synthetic opioid such as tramadol hydrochloride (TRML) and a model pollutant, 4-chlorophenol, in aqueous solution under visible light irradiation (λ > 400 nm). To elucidate mechanisms of photochemical processes, the photocatalytic activity of investigated metalloporphyrins was compared in homo- and heterogeneous systems. The results indicate that impregnation of TiO2 (P25) with functionalized porphyrins can improve its photoactivity. ZnF2PMet@TiO2 exhibited a superior photocatalytic performance towards TRML degradation. The role of singlet oxygen and hydroxyl radicals in photodegradation processes has been elucidated both for MF2PMet and MF2PMet@TiO2 systems.

 Please wait while we load your content...
Please wait while we load your content...