Thermosensitive nanoplatforms for photothermal release of cargo from liposomes under intracellular temperature monitoring
Abstract
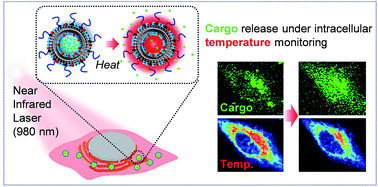
Control of cargo release from nanoscale carriers is an important technology for maximizing the benefits of nanoparticulate drug delivery systems. Herein, we attempt to trigger the release of cargo from liposomes by photothermal conversion of water with a 980 nm near-infrared (NIR) laser. This study examined liposomes of two types formulated by 1,2-dipalmitory-sn-glycero-3-phosphocholine (DPPC) or a mixture of DPPC/cholesterol with an anionic lipid and PEG-lipid as stabilizers encapsulating calcein as a cargo at different ionic strengths. Liposome formulation encapsulating a hypertonic solution with a lipid membrane shows that a gel to liquid-crystalline phase transition at around 40 °C effectively released the cargo from liposomes at temperature above 40 °C with NIR irradiation. Our proof of concept has been further demonstrated in a cancer cell with monitoring the actual “intracellular temperature” using a fluorescent thermosensor. Intracellular thermometry revealed that it was not until the intracellular temperature reached around 40 °C by NIR irradiation that the release of the cargo started gradually, showing good agreement with the result from the extracellular in vitro study. This targeted release of cargo from thermosensitive liposomes based on a photothermal effect using a NIR laser offers a potent nanoscale platform for the on-demand release of drugs in intracellular space with local hyperthermia. The intracellular thermometry facilitates the quantitative monitoring and control of the hyperthermia at the cellular level.

 Please wait while we load your content...
Please wait while we load your content...