Competition between electron doping and short-range scattering in hydrogenated bilayer graphene on hexagonal boron nitride
Abstract
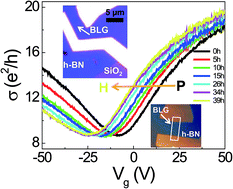
We studied the electron doping of bilayer graphene (BLG) on hexagonal boron nitride (h-BN) by dissociative H2 adsorption. Charge transfer phenomena were investigated by the gate voltage (Vg)-dependent electrical conductivity σ(Vg) and Raman spectroscopy with respect to the H2 exposure. The shift of the charge neutrality point toward the negative Vg region was observed and the charge scattering mechanism was found with the variation of σ(Vg). The charge transfer at the interface as well as the lattice distortion of BLG due to hydrogenation were verified by Raman spectroscopy. From these results, we concluded that the electron doping and short-range scattering in the BLG exposed to high H2 pressure (11 bar) are intrinsic features, which were achieved using a van der Waals interface consisting of BLG and h-BN.

 Please wait while we load your content...
Please wait while we load your content...