The nature of Pd–carbene and Pd–halogen bonds in (bisNHC)PdX2 type catalysts: insights from density functional theory†
Abstract
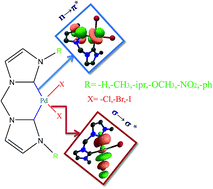
Methylene bridged palladium biscarbene halide complexes have proven to be excellent catalysts in cross coupling reactions and in activation of alkanes. These types of complexes are widely used in decomposition and reductive elimination reactions because of their more stable carbene–Pd bond. The electronic structure and bonding of such methylene bridged palladium biscarbene complexes (LnPdX2) (Ln = NHC) have been investigated using density functional theory. The calculated results reveal that the nature of the substituents as well as the coordinating halide ion determine the strength of the Pd–Ccarbene and Pd–X bonds. These two bonds can be fine-tuned to achieve better catalytic activity through proper substituents. In effect, this can be done by making the Pd–Ccarbene bond much stronger through σ-donation and π-back donation and also by making the Pd–X bond weaker by choosing a suitable halide (X).

 Please wait while we load your content...
Please wait while we load your content...