Generation of silver titania nanoparticles from an Ag–Ti alloy via picosecond laser ablation and their antibacterial activities
Abstract
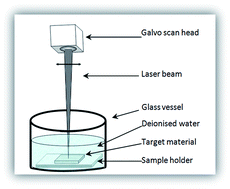
In this work, a bulk Ti/Ag alloy was used, for the first time, to produce Ag–TiO2 compound nanoparticles using picosecond laser ablation in deionised water. Spherical Ag–TiO2 compound nanoparticles with an average size of 31 nm were produced. They were characterised using UV-VIS spectrometry, transmission electron microscopy (TEM), High-Angle Annular Dark-Field-Scanning Transmission Electron Microscopy (HAADF-STEM), Energy Dispersive X-ray Spectroscopy (EDS), X-ray Photoelectron Spectroscopy (XPS) and X-ray Diffraction (XRD) methods to identify the nanoparticle size distribution, morphology, chemical composition, phase and surface properties. It was found that Ag-doped TiO2 nanoparticles were produced. The optical absorption spectra of the Ag–TiO2 compound nanoparticles shifted to longer wavelengths. The antibacterial activity of the Ag–TiO2 compound nanoparticles against Gram-negative Escherichia coli (E. coli) bacteria was examined and compared with those using laser generated Ag and TiO2 nanoparticles and distilled deionised water (as the control). It was found that the antibacterial activity of the Ag–TiO2 compound nanoparticles was better than laser generated TiO2 nanoparticles and chemically produced Ag nanoparticles, and was almost as good as laser generated Ag nanoparticles while Ag–TiO2 was used at a much lower Ag concentrations. The reason behind this is discussed.

 Please wait while we load your content...
Please wait while we load your content...