Mechanism of the Paal–Knorr reaction: the importance of water mediated hemialcohol pathway†
Abstract
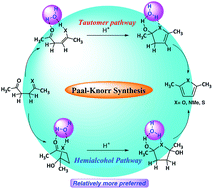
The Paal–Knorr synthesis of furan, pyrrole and thiophene rings is one of the most important methods of generating these very important heterocycles, but the mechanism of this reaction is not well understood. Though several mechanistic paths are suggested, the exact energy requirements of this reaction, the structural features of transition states associated with the cyclization step, have not been established, especially for furan and thiophene synthesis. In this work, we explore the mechanism of the Paal–Knorr method and establish the energy requirements, using quantum chemical methods. The Paal–Knorr reaction to give furans is endergonic by 3.7 kcal mol−1 whereas the same reaction is exergonic for pyrrole and thiophene generation by 16.4 and 15.9 kcal mol−1, using G2MP2 method. The cyclization step is associated with high energy barrier, however, explicit water participation reduces the barrier significantly. For example, under the neutral condition two water mediated pathways – (i) monoenol and (ii) hemiketal, are possible on the reaction leading to furan. The cyclization step in these two pathways require 28.9 and 27.1 kcal mol−1, respectively. The ring formation step becomes highly favorable in the presence of H3O+ with a barrier of only 11.5 kcal mol−1 (solvent phase) from the monoenol to dihydrofuran derivative and 5.5 kcal mol−1 (solvent phase) from hemiketal to dihydroxy dihydrofuran derivative. Similarly, a water mediated pathway involving the intermediacy of hemialcohols has been found to be energetically preferred mechanism for pyrrole and thiophene also.

 Please wait while we load your content...
Please wait while we load your content...