An imidazopyrazine-derived anion for lithium conducting electrolyte application
Abstract
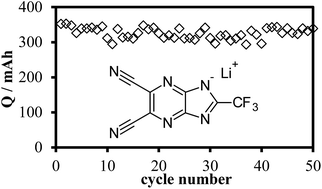
In this work we present a new lithium salt of 4,5-dicyano-2-(trifluoromethyl)imidazopyrazine (LiTDPI) which was designed for use as an electrolyte in lithium-ion cells. It was synthesized and completely characterized by NMR techniques. The salt is thermally stable up to 350 °C and electrochemically stable in carbonate solvents up to +5.1 V vs. Li. Basic electrochemical characterization of this new lithium salt solution shows conductivity of over 2 mS cm−1 at room temperature and a transference number which is higher than the commercial reference salt, LiPF6 (>0.4 in a EC : DMC 1 : 2 ratio mixture). As a proof of concept, short cycling measurements in a graphite half-cell show good capacity (352 mA h g−1) and capacity retention (96% after 50 cycles). The extremely good stability without compromising the performance parameters shows the next leap in progress for tailoring efficient lithium-conducting electrolytes.

 Please wait while we load your content...
Please wait while we load your content...