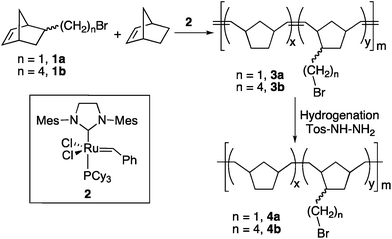
Poly(ω-bromoalkylnorbornenes-co-norbornene) by ROMP-hydrogenation: a robust support amenable to post-polymerization functionalization†
Rodrigo García-Loma and
Ana C. Albéniz*
IU CINQUIMA/Química Inorgánica, Universidad de Valladolid, 47071-Valladolid, Spain. E-mail: albeniz@qi.uva
First published on 10th August 2015
Abstract
The ring opening metathesis copolymerization of norbornene and ω-bromoalkylnorbornenes NB–(CH2)nBr (n = 1, 4) by Grubbs' 2nd generation catalyst, followed by hydrogenation, gives insoluble saturated polynorbornenes (ROMPH-PNBs) that have pendant ω-bromoalkyl chains (4a, b). These materials can be functionalized by nucleophilic substitution of bromide to give a variety of substituted polymers (ROMPH-PNB–(CH2)nNu where Nu = CN, SPh, OOCMe, N3, SnR3. The stannylated polymers were tested in a Pd-catalyzed reaction, the Stille coupling. The azido polynorbornenes ROMPH-PNB–(CH2)nN3 easily undergo the click 1,3-dipolar cycloaddition with alkynes, which could be a useful strategy to anchor other functionalities of interest. The aliphatic nature of the ROMPH-PNB–(CH2)nBr backbone makes robust supports and the presence of the bromo substituent imparts versatility so they are good candidates to be used as a general starting material to anchor a group required for a specific synthetic purpose.
Introduction
Polymeric supports are becoming increasingly important in the context of sustainable synthetic chemistry, since they are used to anchor catalysts or reagents that could be better separated from the products and reused.1 They are also fundamental tools in solid phase organic synthesis.2 Polystyrene and polyethylene glycol are widespread scaffolds for immobilizing a specific functionality. They combine good physical properties and low reactivity and are commercially available, so they can be purchased as conveniently functionalized resins and easily transformed by the chemical practitioner, usually working outside the polymer chemistry area, to attach the reactive group needed. The enormous success of these polymers has obscured other polymeric scaffolds that can be also very useful. In particular, aliphatic saturated carbon chains would make very attractive supports for most applications since, being chemically very robust, they would increase the chances of getting reusable materials. A saturated support is suitable for reactions that may be risky for polystyrene where aromatic and benzylic groups are present, such as aromatic substitutions or radical processes. Few completely saturated polymeric scaffolds have been used in the literature as supports. Polyethylene based FibreCat supported palladium catalysts,3 or imidazolium salts attached to a polyethylene backbone can be found.4 Some examples of polyisobutylene materials have also been reported.1d,5 The availability of aliphatic saturated scaffolds comparable to the well known and versatile halo- or hydroxymethyl functionalized polystyrene resins is very limited.6 We have previously done some work on the vinylic addition copolymerization of haloalkylsubstituted norbornenes and norbornene (VA-PNB, Fig. 1), which gives a saturated polycyclic polymeric material easy to functionalize by nucleophilic substitution of the halogen group.7 These polymers have been used, for example, as precursors of supported stannyl reagents in the Stille reaction,8 or of supported organocatalysts,9 and they can be recycled. With the aim to develop versatile saturated polymeric scaffolds for a variety of applications, we rationalized that a halo-substituted polymer obtained by ring opening metathesis polymerization of the above-mentioned norbornenyl monomers (ROMP-PNB, Fig. 1) could be an excellent precursor to obtain a variety of materials by post-polymerization reactions.10 However, ROMP leads to unsaturated polymer skeletons which contain double bonds as potential reactive centers, so the support may be unstable for certain applications such as catalysis. A way to avoid this drawback is reduction of the polymeric main chain to a saturated one, and this can be achieved by stoichiometric reagents such as hydrazides,11 or by metal catalyzed hydrogenation (ROMPH-PNB, Fig. 1).12 The ROMP-hydrogenated polynorbornene main chain is polycyclic, but the cyclopentenyl units are linked by ethyl groups so, overall, the main chain is expected to be more flexible that the vinylic addition polymer. It has also good optical properties suitable for industrial applications.13 Both types of polynorbornenes (VA-PNB and ROMPH-PNB) have the advantage of being saturated robust scaffolds, so the reactive center lays exclusively on the supported functionality of choice.Ring opening metathesis polymerization of cyclic olefins is applicable to different types of substituted monomers since the reaction tolerates the presence of many functional groups.14 Among those cyclic olefins the strained norbornene is a convenient monomer due to its easy polymerization and many different substituted norbornenes have been polymerized to ROMP-PNB materials with pendant groups that introduce interesting physical properties,12b,15 or have a use as catalysts,16 or reagent support.1d,17 As mentioned before, all these materials have an unsaturated polymeric main chain but few attempts have been made at transforming them on the saturated analogue. Clapham, Janda et al. described a saturated ROMPH polynorbornene resin by ROM emulsion copolymerization of norbornene, 5-hydroxymethyl norbornene and a norbornenyl crosslinker.18 The hydrogenation of the ROMP material was achieved under very harsh conditions, and they used the saturated resin in solid phase organic synthesis by functionalization of the hydroxo group in the polymer. They demonstrated that the saturated ROMPH resin is more resistant than the common polystyrene beads to solid phase Friedel Crafts acylation reactions and gives better yields. Despite this potential advantage, the use of ROMPH scaffolds has not been extended. A reason for that may be that some functional groups on the ROMP polymer may not survive the reduction conditions that lead to a saturated main chain. Thus, the combination of a saturated inert support with a given substitution pattern may not be accessible by direct polymerization of the functionalized monomer and subsequent hydrogenation. The introduction of the target groups by post-polymerization functionalization of a suitable and general saturated scaffold may be more convenient.19 We describe here new saturated polymeric materials obtained by ROMP of norbornene and ω-bromoalkyl norbornenes followed by hydrogenation. These materials have pendant ω-bromoalkyl chains which can be functionalized in a variety of ways and can be used as general precursors to support a specific group of interest. It is noteworthy that, even if a large variety of substituted norbornenes have been polymerized by ROMP, halogenated or haloalkyl substituted norbornenes have rarely been used as monomers and only a few examples have been reported.20
Experimental section
Materials and general considerations
NMR spectra in solution were recorded at 293 K using Bruker AV-400 and Agilent MR-500 and MR-400 instruments. Chemical shifts (δ) are reported in ppm and referenced to SiMe4 (1H and 13C) and CFCl3 (19F) and SnMe4 (119Sn). The solid state NMR spectra were recorded at 293 K under magic angle spinning (MAS) in a Bruker AV-400 spectrometer using a Bruker BL-4 probe with 4 mm diameter zirconia rotors spinning at 8 kHz. 13C CP MAS NMR spectra were measured at 100.61 MHz and recorded with proton decoupling (tppm), with a 90° pulse length of 4.5 μs and a contact time of 3 ms and recycle delay of 3 s. The 13C NMR spectra were referred to glycine (CO signal at 176.1 ppm). IR spectra were collected on the solid samples using a Perkin-Elmer FT/IR SPECTRUM FRONTIER spectrophotometer with CsI + ATR diamond accessory. The bromo content in the polymers was determined by oxygen-flask combustion of a sample and analysis of the residue by mercurimetric titration of the bromide.21 The ratios NB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (x/y, Scheme 1) in the ROMP-PNB copolymers were determined from these values. Considering that [Mw(1) + x/y Mw(NB)] g of copolymer contains 1 mol of Br, x/y is given by the formula x/y = [1000 − α Mw(1)]/[α Mw(NB)] where α = mmol Br per g copolymer, Mw(1) and Mw(NB) molecular weight of monomers 1 and norbornene. Size exclusion chromatography (SEC) was carried out on a Waters SEC system using a three-column bed (Styragel 7.8 × 300 mm columns: 50–100
1 (x/y, Scheme 1) in the ROMP-PNB copolymers were determined from these values. Considering that [Mw(1) + x/y Mw(NB)] g of copolymer contains 1 mol of Br, x/y is given by the formula x/y = [1000 − α Mw(1)]/[α Mw(NB)] where α = mmol Br per g copolymer, Mw(1) and Mw(NB) molecular weight of monomers 1 and norbornene. Size exclusion chromatography (SEC) was carried out on a Waters SEC system using a three-column bed (Styragel 7.8 × 300 mm columns: 50–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 D, 5000–500
000 D, 5000–500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 D and 2000–4
000 D and 2000–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 D) and Waters 410 differential refractometer. SEC samples were run in CHCl3 at 313 K and calibrated to polystyrene standards. Residual tin in the Stille coupling product was determined by ICP-MS using Agilent 7500i equipment; the sample was dissolved in a mixture of HNO3/H2SO4 = 7
000 D) and Waters 410 differential refractometer. SEC samples were run in CHCl3 at 313 K and calibrated to polystyrene standards. Residual tin in the Stille coupling product was determined by ICP-MS using Agilent 7500i equipment; the sample was dissolved in a mixture of HNO3/H2SO4 = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 using an ETHOS SEL Milestone microwave oven. Solvents used in the reactions and to prepare solutions of the reagents were dried using a Solvent Purification System (SPS PS-MD-5) or distilled from appropriate drying agents, de-oxygenated and kept under nitrogen, prior to use. Norbornene, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), tetrabutylammonium cyanide, sodium azide, thiophenol, dimethylacetylenedicarboxylate, the terminal alkynes, the organic halides and the ruthenium complex 2 were purchased from Aldrich, Acros, Merck or Lancaster. ω-Bromoalkyl norbornenes 1,7 and SnHBu2(p-C6H4OMe),22 were prepared according to procedures reported in the literature.
3 using an ETHOS SEL Milestone microwave oven. Solvents used in the reactions and to prepare solutions of the reagents were dried using a Solvent Purification System (SPS PS-MD-5) or distilled from appropriate drying agents, de-oxygenated and kept under nitrogen, prior to use. Norbornene, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), tetrabutylammonium cyanide, sodium azide, thiophenol, dimethylacetylenedicarboxylate, the terminal alkynes, the organic halides and the ruthenium complex 2 were purchased from Aldrich, Acros, Merck or Lancaster. ω-Bromoalkyl norbornenes 1,7 and SnHBu2(p-C6H4OMe),22 were prepared according to procedures reported in the literature.
Synthesis of ROMP-PNB–NBCH2Br (3a, x/y = 4.85)
A solution of norbornene in CH2Cl2 (1.22 mL, 3.49 M, 4.26 mmol) was mixed with 1a (0.15 mL, 1.07 mmol) and CH2Cl2 (6 mL) under a nitrogen atmosphere. A solution of 2 in CH2Cl2 (0.24 mL, 8.96 × 10−4 M, 2.1 × 10−4 mmol) was added dropwise and CH2Cl2 was added to get a total volume of 8 mL. The reaction mixture was stirred for 4 h at room temperature. The solution became darker and increased its viscosity as the reaction progressed. The reaction mixture was poured onto MeOH and a solid precipitated, which was crushed, stirred with MeOH, filtered, washed with MeOH (3 × 15 mL) and air-dried. Off-white gum-like solid. 0.52 g, 86% yield (Chart 1). 1H NMR (CDCl3, 500.13 MHz): δ 5.35 (b, H2trans + H3trans, NB + 1a), 5.20 (b, H2cis + H3cis, NB + 1a), 3.49, 3.34 (b, H8cis + H8′cis), 3.40, 3.24 (b, H8trans + H8′trans), 3.06 (b, H1 + H4, 1a), 2.79 (b, H1cis + H4cis, NB; H1 + H4, 1a), 2.44 (b, H1trans + H4trans, NB; H1 + H4 + H5, 1a), 2.05 (b, H6 + H7, 1a), 1.85 (b, H7, NB), 1.80, 1.35 (b, H5 + H6, NB), 1.25 (b, H6′ + H7′, 1a), 1.03 (m, H7′, NB). 13C NMR (CDCl3, 125.76 MHz): δ 134.0, 133.9, 133.8, 133.7 (C2cis + C3cis, NB + 1a), 133.1, 133.0, 132.8 (C2trans + C3trans, NB + 1a), 46.0 (C5, 1a), 43.6, 43.3, 42.9 (C1trans + C4trans, NB), 42.3 (C7, NB), 41.5 (C6 or C7, 1a), 42.7, 41.8, 40.4, 37.1 (C1 + C4, 1a), 39.8 (C6 or C7, 1a), 38.8, 38.6 (C1cis + C4cis, NB), 38.6 (C8cis), 36.8 (C8trans), 33.3, 33.1, 32.6, 32.5 (C5 + C6, NB). IR (neat, cm−1), ν(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH): 972 (s), 750 (m); ν(C–Br): 640 (m). The copolymer contains 124.07 mg (1.553 mmol) of Br per g of polymer, which indicates a monomer ratio in the polymer of NB
CH): 972 (s), 750 (m); ν(C–Br): 640 (m). The copolymer contains 124.07 mg (1.553 mmol) of Br per g of polymer, which indicates a monomer ratio in the polymer of NB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a = 4.85 (x/y = 4.85).
1a = 4.85 (x/y = 4.85).
The polymers collected in Tables 1–3 were prepared in the same way by just using the corresponding bromoalkylnorbornene and changing the amount of norbornene or CTA (Table 2) to reach the appropriate ratio in the feed, and the catalyst amount used (Table 3) (Chart 1).
| Entry | Monomer | Feed ratiob NB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Yieldc (%) | Copolymerd, x/y | mmol Br per g in 3 |
|---|---|---|---|---|---|
| a The reactions were carried out under nitrogen in CH2Cl2 (total volume 8 mL) for 4 h at room temperature using 0.7 mmol of 1a or 0.5 mmol of 1b.b Molar ratio in the feed.c Yields are referred to the total monomer mass.d x/y ratio was determined by quantitative analyses of the bromo content in the polymer (see Experimental section). | |||||
| 1 | 1a | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 5000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 | 3a, 0 | 5.3 |
| 2 | 1a | 5000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 5000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
93 | 3a, 1.05 | 3.5 |
| 3 | 1a | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 5000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
84 | 3a, 2.8 | 2.2 |
| 4 | 1a | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 5000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
91 | 3a, 6.2 | 1.3 |
| 5 | 1a | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 5000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
97 | 3a, 11.3 | 0.8 |
| 6 | 1b | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 5000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
93 | 3b, 0 | 4.4 |
| 7 | 1b | 5000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 5000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94 | 3b, 1.35 | 2.8 |
| 8 | 1b | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 5000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96 | 3b, 3.1 | 1.9 |
| 9 | 1b | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 5000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96 | 3b, 5.1 | 1.4 |
| 10 | 1b | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 5000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
96 | 3b, 10.8 | 0.8 |
| Entry | Monomer | NB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CTA CTA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2b 2b |
3, x/yc | Yieldd (%) | Mn (x 10−3) | Mw/Mn |
|---|---|---|---|---|---|---|
| a CTA: ethyl vinyl ether. The reactions were carried out under nitrogen in CH2Cl2 (total volume 8 mL) for 4 h at room temperature using 0.7 mmol of 1a or 0.5 mmol of 1b.b Molar ratio in the feed.c x/y ratio was determined by quantitative analyses of the bromo content in the polymer (see Experimental section).d Yields are referred to the total monomer mass. | ||||||
| 1 | 1a | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 5000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3a, 0 | 74 | 22.2 | 2.12 |
| 2 | 1b | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 5000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3b, 0 | 83 | 36.8 | 2.23 |
| 3 | 1a | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 5000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3a, 4.81 | 91 | 29.1 | 3.47 |
| 4 | 1b | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 5000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3b, 4.88 | 93 | 54.2 | 6.57 |
| Entry | Monomer | NB : 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2b 2b |
Reaction time (min) | Mn (×10−3) | Mw/Mn | trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis ratioc cis ratioc |
|---|---|---|---|---|---|---|
| a The reactions were carried out under nitrogen in CH2Cl2 (total volume 8 mL) at room temperature using 0.1 mL of 1 (0.09 M for 1a and 0.07 M for 1b); the yields obtained range from 65 to 85% in the homopolymerization reactions and >89% for the copolymerizations.b Molar ratio in the feed.c Alkene trans/cis ratio in the polymer was determined by 1H NMR.d Composition of copolymers x/y: 1 (entry 7), 1.1 (entry 8), 0.95 (entry 9), 1.9 (entry 10). | ||||||
| 1 | 1a | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15 | 13.9 | 2.80 | 2.23 |
| 2 | 1a | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
30 | 14.3 | 2.80 | 2.35 |
| 3 | 1a | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 | 6.6 | 3.26 | 2.61 |
| 4 | 1a | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
240 | 3.9 | 3.16 | 2.84 |
| 5A | 1a | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40 | 14.9 | 2.83 | 2.28 |
| 5B | 1a | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
240 | 7.1 | 2.87 | 2.45 |
| 6 | 1a | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
240 | 24.8 | 2.09 | 2.31 |
| 7d | 1a | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
240 | 3.8 | 3.5 | 1.79 |
| 8d | 1a | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
240 | 38.5 | 3.39 | 1.21 |
| 9d | 1b | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
240 | 5.4 | 6.57 | 1.23 |
| 10d | 1a | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
240 | 4.52 | 4.57 | 1.50 |
 | ||
| Chart 1 Numbering scheme for the repeating norbornene-derived units in both the ROMP and ROMPH polymers (only the ROMPH repeating 1a or 1b units are shown). | ||
ROMP-PNBCH2Br (3a, x/y = 0)
Off-white gum-like solid. 87% yield. 1H NMR (CDCl3, 500.13 MHz): δ 5.37 (b, H2trans + H3trans), 5.22 (b, H2cis + H3cis), 3.46, 3.30 (b, H8cis + H8′cis), 3.36, 3.26 (b, H8trans + H8′trans), 3.05, 2.83, 2.72, 2.49 (b, H1 + H4), 2.44 (b, H5), 2.07 (b, H6 + H7), 1.23 (b, H6′ + H7′). 13C NMR (CDCl3, 125.76 MHz): δ 135.9, 133.1, 131.4, 128.7 (C2cis + C3cis), 135.7, 134.1, 130.6, 129.1 (C2trans + C3trans) 45.9 (C5), 45.5, 45.4, 45.2, 42.0, 41.8, 41.5, 39.9, 37.4, 37.3, 37.1 (C1 + C4), 40.8, 40.2, 40.1, 39.7, 39.5, 38.9, 38.5 (C6 + C7), 36.7 (C8). IR (neat, cm−1), ν(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH): 968 (s), 746 (m); ν(C–Br): 635 (m). It contains 399.70 mg (5 mmol) of Br per g of polymer.
CH): 968 (s), 746 (m); ν(C–Br): 635 (m). It contains 399.70 mg (5 mmol) of Br per g of polymer.
ROMP-PNB–NB(CH2)4Br (3b, x/y = 5.74)
A monomer feed ratio NB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1b = 4
1b = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used. Off-white gum-like solid. 97% yield. 1H NMR (CDCl3, 500.13 MHz): δ 5.35 (b, H2trans + H3trans, NB + 1b), 5.21 (b, H2cis + H3cis, NB + 1b), 3.39 (b, H11), 2.93 (b, H1 + H4, 1b), 2.79 (b, H1cis + H4cis, NB; H1 + H4, 1b), 2.54 (b, H1 + H4, 1b), 2.44 (b, H1trans + H4trans, NB; H1 + H4, 1b), 1.99 (H7, 1b), 1.86, 1.80 (b, H5 or H6 + H7, NB; H5 + H8 + H10, 1b), 1.36 (b, H5 or H6, NB; H6 + H9, 1b), 1.14 (b, H6′ + H7′, 1b), 1.03 (m, H7′, NB). 13C NMR (CDCl3, 125.76 MHz): δ 134.2, 134.1, 134.0, 133.9 (C2cis + C3cis, NB + 1b), 133.3, 133.1, 133.0 (C2trans + C3trans, NB + 1b), 43.6, 43.3 (C1trans + C4trans, NB), 42.9 (C5, 1b), 42.2 (C7, NB), 41.5 (C7, 1b), 45.4, 41.0, 40.4, 39.8, 37.6 (C1 + C4, 1b), 38.8, 38.6 (C1cis + C4cis, NB), 34.2 (C11), 33.3, 33.1, 32.5, 32.4 (C5 + C6 + C10, NB), 31.0 (C6, 1b), 27.4 (C9), 27.2 (C8). IR (neat, cm−1), ν(
1 was used. Off-white gum-like solid. 97% yield. 1H NMR (CDCl3, 500.13 MHz): δ 5.35 (b, H2trans + H3trans, NB + 1b), 5.21 (b, H2cis + H3cis, NB + 1b), 3.39 (b, H11), 2.93 (b, H1 + H4, 1b), 2.79 (b, H1cis + H4cis, NB; H1 + H4, 1b), 2.54 (b, H1 + H4, 1b), 2.44 (b, H1trans + H4trans, NB; H1 + H4, 1b), 1.99 (H7, 1b), 1.86, 1.80 (b, H5 or H6 + H7, NB; H5 + H8 + H10, 1b), 1.36 (b, H5 or H6, NB; H6 + H9, 1b), 1.14 (b, H6′ + H7′, 1b), 1.03 (m, H7′, NB). 13C NMR (CDCl3, 125.76 MHz): δ 134.2, 134.1, 134.0, 133.9 (C2cis + C3cis, NB + 1b), 133.3, 133.1, 133.0 (C2trans + C3trans, NB + 1b), 43.6, 43.3 (C1trans + C4trans, NB), 42.9 (C5, 1b), 42.2 (C7, NB), 41.5 (C7, 1b), 45.4, 41.0, 40.4, 39.8, 37.6 (C1 + C4, 1b), 38.8, 38.6 (C1cis + C4cis, NB), 34.2 (C11), 33.3, 33.1, 32.5, 32.4 (C5 + C6 + C10, NB), 31.0 (C6, 1b), 27.4 (C9), 27.2 (C8). IR (neat, cm−1), ν(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH): 971 (s), 740 (m); ν(C–Br): 637 (w), 557 (w). It contains 103.69 mg (1.30 mmol) of Br per g of polymer, which indicates a monomer ratio in the polymer of NB
CH): 971 (s), 740 (m); ν(C–Br): 637 (w), 557 (w). It contains 103.69 mg (1.30 mmol) of Br per g of polymer, which indicates a monomer ratio in the polymer of NB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1b = 5.74
1b = 5.74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
ROMP-PNB(CH2)4Br (3b, x/y = 0)
Off-white gum-like solid. 93% yield. 1H NMR (CDCl3, 500.13 MHz): δ 5.30 (b, H2trans, H3trans), 5.20 (b, H2cis, H3cis), 3.40 (b, H11), 2.92, 2.77, 2.57, 2.44 (b, H1 + H4), 1.91 (b, H5 + H7) 1.84 (b, H8 + H10), 1.38 (b, H6 + H9), 1.14 (b, H6′ + H7′). 13C NMR (CDCl3, 125.76 MHz): δ 134.8, 133.5, 131.9, 130.4 (C2cis + C3cis), 134.8, 134.5, 131.2, 130.8 (C2trans + C3trans), 45.8, 45.4, 42.4, 40.9, 37.5 (C1 + C4), 43.5 (C5), 40.3 (C7), 34.2 (C11), 33.2 (C10), 31.1 (C6), 27.4 (C9), 27.2 (C8). IR (neat, cm−1), ν(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH): 969 (s), 736 (m); ν(C–Br): 641 (s), 558 (s). It contains 309.70 mg (3.9 mmol) of Br per g of polymer.
CH): 969 (s), 736 (m); ν(C–Br): 641 (s), 558 (s). It contains 309.70 mg (3.9 mmol) of Br per g of polymer.
Synthesis of ROMPH-PNB–NBCH2Br (4a)
Chlorobenzene (450 mL) was added to a mixture of ROMP-PNB–NBCH2Br (3a, x/y = 7.9, 6.76 g, 64.63 double bond equiv.) and p-toluensulfonylhydrazide (48.19 g, 258.93 mmol) under a nitrogen atmosphere. The polymer swelled in the solvent after 10–15 min at room temperature. The reaction mixture was refluxed for 5 h and the formation of foam was observed. After this time, the mixture was poured onto MeOH (300 mL) and the beige gum-like solid was filtered, washed with MeOH (4 × 50 mL) and air-dried. 6.65 g, 96% yield. If needed, the polymer can be swollen in CH2Cl2 and then precipitated onto MeOH to facilitate the elimination of residual chlorobenzene. 1H NMR (CDCl3, 400.13 MHz): δ 3.52 (m, H8), 3.27 (m, H8′), 2.35 (b, H5endo, 1a), 2.04 (b, H6endo, 1a), 1.91 (b, H7, NB; H1 + H5exo + H7, 1a), 1.71 (b, H1 + H4 + H5 or H6, NB; H4 + H6exo, 1a), 1.42 (b, H2 + H6′exo, 1a), 1.26 (b, H2 + H3, NB; H3, 1a), 1.13 (b, H5 or H6, NB), 1.07 (b, H2′ + H6′endo, 1a), 0.88 (m, H7′, 1a), 0.61 (m, H7′, NB). 13C NMR (CDCl3, 100.61 MHz): δ 44.7 (C5, 1a), 42.4 (C1, 1a), 40.9 (C7, NB), 40.5 (C1 + C4, NB), 39.1 (C4, 1a), 38.8 (C7, 1a), 38.6 (C6, 1a), 37.6 (C8), 36.2, 35.6 (C3, 1a), 35.9 (C2 + C3, NB), 31.9 (C5 + C6, NB), 29.5 (C2, 1a). IR (neat, cm−1), ν(C–Br): 638 (m). The copolymer contains 79.89 mg (1 mmol) of Br per g of polymer. All the saturated ROMPH polymers were prepared in the same way.ROMPH-PNBCH2Br (4a)
Prepared from 3a, x/y = 0. Off-white gum-like solid. 54% yield. 1H NMR (CDCl3, 500.13 MHz): δ 3.51 (m, H8), 3.27 (m, H8′), 2.36 (b, H5endo), 2.05 (b, H6endo), 1.95 (b, H1 + H5exo + H7), 1.79 (b, H4), 1.71 (b, H6exo), 1.50, 1.44 (b, H2 + H6′exo), 1.32 (b, H3), 1.07 (b, H2′ + H6′endo), 0.89 (b, H7′). 13C NMR (CDCl3, 100.61 MHz): 46.9 (C5exo), 44.4 (C5endo), 42.5, 42.1 (C1), 39.6 (C8exo) 38.8 (C4), 38.6 (C7), 38.4 (C6endo), 37.7 (C6exo), 37.2 (C8endo), 35.9, 35.7 (C3), 29.2 (C2). IR (neat, cm−1), ν(C–Br): 638 (m).ROMPH-PNB–NB(CH2)4Br (4b)
Prepared from 3b, x/y = 10.48. Off-white gum-like solid. 88% yield. 1H NMR (CDCl3, 400.13 MHz): δ 3.41 (b, H11), 1.88 (b, H7, NB; H7exo + H7endo + H10, 1b), 1.71 (b, H1 + H4 + H5 or H6, NB; H1 + H4 + H5 + H8, 1b), 1.45–1.20 (b, H2 + H3, NB; H2 + H3 + H6 + H9, 1b), 1.13 (b, H5 or H6, NB; H6′, 1b), 0.82 (b, H7′endo, 1b), 0.63 (b, H7′, NB; H7′exo, 1b). 13C NMR (CDCl3, 100.61 MHz): δ 44.7 (C5exo, 1b), 43.0, 42.2, 41.8 (C1 + C4, 1b), 40.9 (C7, NB; C7exo, 1b), 40.5 (C1 + C4, NB), 39.2 (C5endo, 1b), 38.7 (C7endo, 1b), 38.3 (C8, 1b), 36.6 (C2 + C3, 1b), 35.9 (C2 + C3, NB), 34.2 (C11), 33.3 (C10), 31.9 (C5 + C6, NB), 30.2, 30.0 (C6exo + C6endo, 1b), 27.3 (C9). IR (neat, cm−1), ν(C–Br): 651(m), 564 (m). The copolymer obtained contains 55.32 mg (0.69 mmol) of Br per g of polymer.ROMPH-PNB(CH2)4Br (4b)
Prepared from 3b, x/y = 0: off-white gum-like solid. 72% yield. 1H NMR (CDCl3, 500.13 MHz): δ 3.42 (t, H11), 1.97 (b, H7exo), 1.90–1.65 (m, H1 + H4 + H5endo + H7endo + H8 + H10), 1.47 (b, H9), 1.40–1.20 (b, H2 + H3 + H5exo + H6exo + H6endo H9′), 1.07 (m, H6′endo), 0.94 (b, H6′exo), 0.81 (b, H7′endo), 0.67 (b, H7′exo). 13C NMR (CDCl3, 100.61 MHz): δ 44.7 (C5exo), 42.7, 42.2, 41.7 (C1 + C4), 40.8 (C7exo), 39.3 (C5endo), 38.8 (C7endo), 38.3 (C8), 36.7, 36.5 (C2 + C3), 34.3 (C11), 33.3 (C10), 30.1 (C6exo + C6endo), 27.3 (C9). IR (neat, cm−1), ν(C–Br): 647 (m), 563 (s).Determination of reactivity ratios
Copolymerizations of norbornene and 1a were carried out at monomer feed ratios f = NB/1a = 0.94, 1.87, 3.80 and 7.61 using 0.10 mL (0.72 mmol) of 1a in each experiment and following the conditions of Table 1. After 60 min the reactions were quenched by pouring the mixture onto methanol. The composition of the copolymers was determined by quantitative analysis of the bromo content in the material. The composition data of the copolymers (F) obtained for the copolymerization at different monomer feed ratios (f) were fitted to the Finemann-Ross equation.23 The reactivity ratios for the copolymerization of NB and 1b were determined in a similar way. Details can be found in the ESI.†Synthesis of ROMPH-PNB–NBCH2CN (5a)
Polymer 4a (0.2 g, 0.28 mmol of Br), tetrabutylammonium cyanide (0.12 g, 0.42 mmol) and dry THF (35 mL) were mixed in a flask under nitrogen. The polymer swelled and the reaction mixture was stirred for 24 h at reflux. The mixture was poured onto MeOH (100 mL) and a white gum-like solid precipitated, which was filtered, washed with MeOH (3 x 15 mL), air-dried and kept overnight at 35 °C in vacuo. 0.18 g, 98% yield. 1H NMR (CDCl3, 500.13 MHz): δ 2.37 (m, H8), 2.14 (m, H8′), 1.89 (m, H7, NB; H1 + H7, NB–CH2CN), 1.71 (b, H1 + H4 + H5 or H6, NB; H4 + H6, NB–CH2CN), 1.26 (b, H2 + H3, NB; H2 or H3, NB–CH2CN), 1.13 (b, H5 or H6, NB; H5, NB–CH2CN), 0.87 (m, H7′, NB–CH2CN), 0.63 (m, H7′, NB). 13C NMR (CDCl3, 100.61 MHz): δ 120.4 (CN), 40.9 (C7, NB), 40.5 (C1 + C4, NB), 38.4 (C5, NB–CH2CN), 35.7 (C2 + C3, NB), 31.9 (C5 + C6, NB), 29.7 (C2 or C3, NB–CH2CN), 19.6 (C8). IR (neat, cm−1), ν(CN): 2254 (w). The copolymer contains 3.32 mg (0.041 mmol) of Br per g, which indicates a 97% of bromine substitution.Synthesis of ROMPH-PNB–NBCH2OCOMe (6a)
Polymer 4a (0.2 g, 0.28 mmol of Br), acetic acid (0.040 mL, 0.70 mmol), DBU (0.10 mL, 0.70 mmol) and toluene (30 mL) were mixed in a flask under nitrogen. The reaction was stirred for 24 h at reflux. The solution was poured onto MeOH (100 mL) and a white gum-like solid precipitated. The solid was filtered, washed with MeOH (3 × 15 mL), air-dried and kept overnight at 35 °C in vacuo. 0.16 g, 85% yield. 1H NMR (CDCl3, 500.13 MHz): δ 4.09 (b, H8), 3.88 (b, H8′), 2.22 (b, H5, NB–CH2OCOMe), 2.04 (b, Me), 1.89 (b, H7, NB; H1 + H7, NB–CH2OCOMe), 1.71 (b, H1 + H4 + H5 or H6, NB; H4 + H6, NB–CH2OCOMe), 1.26 (b, H2 + H3, NB; H2 or H3, NB–CH2OCOMe), 1.13 (b, H5 or H6, NB), 0.86 (b, H7′, NB–CH2OCOMe), 0.62 (m, H7′, NB). 13C NMR (CDCl3, 100.61 MHz): δ 170.9 (CO), 66.6 (C8), 41.0 (C7, NB), 40.6 (C1 + C4, NB), 40.2 (C5, NB–CH2OCOMe), 39.3 (C4, NB–CH2OCOMe), 35.9 (C2 + C3, NB), 31.9 (C5 + C6, NB), 29.8 (C2 or C3, NB–CH2OCOMe), 21.3 (Me). IR (neat, cm−1), ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O): 1744 (s); ν(C–O): 1240 (s). The copolymer contains 19.58 mg (0.245 mmol) of Br per g, which indicates 83% of substitution.
O): 1744 (s); ν(C–O): 1240 (s). The copolymer contains 19.58 mg (0.245 mmol) of Br per g, which indicates 83% of substitution.
Synthesis of ROMPH-PNB–NBCH2SPh (7a)
Polymer 4a (0.2 g, 0.28 mmol of Br), thiophenol (0.060 mL, 0.56 mmol), DBU (0.086 mL, 0.56 mmol) and toluene (30 mL) were mixed in a flask under nitrogen. The reaction was stirred for 24 h at reflux. The mixture was poured onto MeOH (100 mL) and a white gum-like solid precipitated. The solid was filtered, washed with MeOH (3 × 15 mL), air-dried and kept overnight at 35 °C in vacuo. 0.20 g, 97% yield. 1H NMR (CDCl3, 500.13 MHz): δ 7.31 (b, Hortho, Hmeta), 7.15 (b, Hpara), 3.08 (dd, J = 11, 5 Hz, H8), 2.69 (t, J = 11 Hz, H8′), 2.15 (b, H5, NB–CH2SPh), 2.03 (m, H6, NB–CH2SPh), 1.90 (m, H7, NB; H1 + H7, NB–CH2SPh), 1.71 (b, H1 + H4 + H5 or H6, NB; H4, NB–CH2SPh), 1.45 (b, H2, NB–CH2SPh), 1.27 (b, H2 + H3, NB; H3, NB–CH2SPh), 1.14 (b, H5 or H6, NB; H6′, NB–CH2SPh), 0.86 (m, H7′, NB–CH2SPh), 0.63 (m, H7′, NB). 13C NMR (CDCl3, 125.76 MHz): δ 137.7 (Cipso), 129.0, 128.9 (Cortho, Cmeta), 125.7 (Cpara), 42.4 (C1, NB–CH2SPh); 41.0 (C7, NB; C5, NB–CH2SPh), 40.6 (C1 + C4, NB), 39.1 (C4, NB–CH2SPh), 38.8 (C7, NB–CH2SPh), 38.7 (C6, NB–CH2SPh). 36.5 (C8), 35.9, 35.7 (C2 + C3, NB; C3, NB–CH2SPh), 31.9 (C5 + C6, NB), 29.9 (C2, NB–CH2SPh). IR (neat, cm−1), 1584 (w), 734 (s), 690 (s), 473 (m). The copolymer contains 3.53 mg (0.044 mmol) of Br per g, which indicates 97% of substitution.Synthesis of ROMPH-PNB–NBCH2N3 (8a)
Polymer 4a (6.5 g, 6.5 mmol of Br) and NaN3 (2.54 g, 39 mmol) were mixed in DMF (200 mL) under nitrogen. The suspension was refluxed for 24 h and then it was poured onto MeOH (200 mL). The solid was filtered, washed with MeOH (3 × 50 mL and 3 × 15 mL), air-dried and kept overnight at 35 °C in vacuo. Off-white spongy solid. 6.12 g, 98% yield. 13C CP-MAS NMR (100.61 MHz): 43.2 (b), 37.9 (b), 32.9 (b). IR (neat, cm−1), ν(N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N): 2091 (vs); ν(N
N): 2091 (vs); ν(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N): 1269 (vs). The polymer contains no Br which indicates 100% of substitution.
N): 1269 (vs). The polymer contains no Br which indicates 100% of substitution.
ROMPH-PNB–NB(CH2)4N3 (8b) was prepared following the same procedure, but using polymer 4b (0.45 g, 0.71 mmol of Br). Ochre spongy polymer. 0.40 g, 96% yield. 13C CP-MAS NMR (100.61 MHz): 42.8 (b), 38.0 (b), 33.2 (b). The copolymer contains no Br, which indicates 100% of substitution. IR (neat, cm−1), ν(N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N): 2090 (vs); ν(N
N): 2090 (vs); ν(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N): 1254 (vs).
N): 1254 (vs).
Synthesis of ROMPH-PNB–NB(CH2)4SnBu2(p-C6H4OMe) (9b)
Diisopropylamine (4.23 mL, 29.94 mmol) was dissolved in THF (40 mL) under a nitrogen atmosphere and set at −78 °C. Butyl lithium (18.71 mL, 1.6 M, 29.94 mmol) was added dropwise and stirred for 90 min at −78 °C. Dibutylanisyltinhydride (10.21 g, 29.94 mmol) dissolved in THF (5 mL) was added dropwise and stirred during 60 min at −78 °c. The solution turned dark yellow. Polymer 4b (4.90 g, 6.67 mmol of Br) was swelled in THF (120 mL) under a nitrogen atmosphere and cooled to −78 °C. The solution of LiSnBu2(p-C6H4OMe) was added to the polymer via cannula. The mixture was stirred for 24 h and the temperature was slowly risen during this time to room temperature. The mixture was poured onto MeOH and a solid precipitated; it was filtered, crushed, washed with MeOH (3 × 50 mL and 3 × 20 mL), air-dried and kept overnight in vacuo at 35 °C. Off-white gum-like solid. 6.08 g, 92% yield. 1H NMR (CDCl3, 500.13 MHz): δ 7.37 (m, Hortho), 6.90 (m, Hmeta), 3.80 (b, OCH3), 1.88 (b, H7, NB + NB(CH2)4SnR2R′), 1.71 (b, H1 + H4 + H5 or H6, NB; H1 + H4 + H5, NB(CH2)4SnR2R′), 1.54 (b, CH2CH2Sn), 1.33 (m, CH2(CH2)2Sn), 1.26 (b, H2 + H3, NB + NB(CH2)4SnR2R′), 1.13 (b, H5 or H6, NB), 1.02 (m, CH2Sn), 0.88 (t, J = 7.3 Hz, CH3(Bu); H7′, NB(CH2)4SnR2R′), 0.62 (m, H7′, NB). 13C NMR (CDCl3, 100.61 MHz): δ 137.4 (Cortho), 114.1 (Cmeta), 55.3 (OCH3), 42.0 (C5, NB(CH2)4SnR2R′), 41.0 (C7, NB + NB(CH2)4SnR2R′), 40.6 (C1 + C4, NB + NB(CH2)4SnR2R′), 35.9 (C2 + C3, NB + NB(CH2)4SnR2R′), 31.9 (C5 + C6, NB), 29.3 (CH2CH2Sn), 27.0 (CH2(CH2)2Sn), 13.9 (CH3(Bu)), 9.8 (CH2Sn). 119Sn NMR (CDCl3, 149.21 MHz): δ −42.08. IR (neat, cm−1), 1587 (m), 1494 (m), 1276 (s), 1242 (s), 1180 (s), 1035 (m), 805 (m), 514 (m). The copolymer contains no Br, which indicates 100% of substitution.Synthesis of ROMPH-PNB–NBCH2triazole (10a)
Polymer 8a (0.2 g, 0.21 mmol of azide), dimethyl acetylenedicarboxylate (0.1 mL, 0.82 mmol) and toluene (12 mL) were mixed in a flask. The reaction mixture was stirred at reflux for 8 h, and poured onto MeOH. The solid was filtered, washed with MeOH (3 × 15 mL), air-dried and kept overnight at 35 °C in vacuo. Ochre-white solid. 0.21 g, 95% yield. 13C CP-MAS NMR (100.61 MHz): δ 161.0 (OCOR); 140.2, 130.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 53.6 (OCH3); 42.9, 37.8, 33.1 (polymeric backbone). IR (neat, cm−1), ν(C
C); 53.6 (OCH3); 42.9, 37.8, 33.1 (polymeric backbone). IR (neat, cm−1), ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O): 1734 (vs); ν(C–O st. as): 1216 (s); ν(C–O st. sim): 1059 (m).
O): 1734 (vs); ν(C–O st. as): 1216 (s); ν(C–O st. sim): 1059 (m).
General synthesis of ROMPH-PNB–NBCH2triazoles 11a–15a
Polymer 8a (0.2 g, 0.208 mmol of azide), CuI (0.0779 g, 0.41 mmol), DIPEA (1.78 mL, 10.2 mmol), the corresponding alkyne (0.82 mmol) and THF (20 mL) were mixed in a flask and stirred at the appropriate temperature for 24 h under nitrogen (see Table 6). The resulting mixture was poured onto MeOH (20 mL) and a cloudy yellow suspension was obtained. Aqueous NH3 (30 mL, 32% w/w) was added, and the mixture turned blue. The solid was filtered, washed with 16.7 M aqueous NH3 (3 × 15 mL), H2O (3 × 15 mL), a H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 1
MeOH = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture (3 × 15 mL), air-dried and kept overnight at 35 °C in vacuo. The product was a solid insoluble in most common organic solvents.
1 mixture (3 × 15 mL), air-dried and kept overnight at 35 °C in vacuo. The product was a solid insoluble in most common organic solvents.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR), 120.0 (C
CR), 120.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR); 43.0, 37.8, 32.9 (polymeric backbone + tBu). IR (neat, cm−1): 1223 (m), 1045 (m).
CR); 43.0, 37.8, 32.9 (polymeric backbone + tBu). IR (neat, cm−1): 1223 (m), 1045 (m).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR), 129.8 (C
CR), 129.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C cyclohexenyl), 126.8–112.7 (C
C cyclohexenyl), 126.8–112.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C cyclohexenyl; C
C cyclohexenyl; C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR); 42.9, 37.8, 33.0 (polymeric backbone + aliphatic cyclohexenyl). IR (neat, cm−1): 1044 (m).
CR); 42.9, 37.8, 33.0 (polymeric backbone + aliphatic cyclohexenyl). IR (neat, cm−1): 1044 (m).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR), 138.5–124.5 (CPh + C
CR), 138.5–124.5 (CPh + C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR); 43.0, 37.8, 33.1 (polymeric backbone). IR (neat, cm−1): 1636 (w), 758 (s), 692 (s).
CR); 43.0, 37.8, 33.1 (polymeric backbone). IR (neat, cm−1): 1636 (w), 758 (s), 692 (s).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR), 136.2 (Cipso), 130.9 (Cpara), 126.7 (C
CR), 136.2 (Cipso), 130.9 (Cpara), 126.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR, Cortho, Cmeta), 124.5 (CF3); 42.9, 37.8, 33.1 (polymeric backbone). 19F MAS NMR (376.5 MHz): δ −60.4. IR (neat, cm−1), ν(C–F st.):1321 (vs); 1171 (s), 1129 (vs), 1059 (s).
CR, Cortho, Cmeta), 124.5 (CF3); 42.9, 37.8, 33.1 (polymeric backbone). 19F MAS NMR (376.5 MHz): δ −60.4. IR (neat, cm−1), ν(C–F st.):1321 (vs); 1171 (s), 1129 (vs), 1059 (s).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR), 122.4–110.6 (C
CR), 122.4–110.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR, Cortho, NC–Cipso), 114.7 (Cmeta), 56.5 (OCH3); 42.8, 37.8, 33.0 (polymer backbone). IR (neat, cm−1): 1500 (m), 1248 (s), 1174 (m), 1031 (m), 828 (m), 541 (m).
CR, Cortho, NC–Cipso), 114.7 (Cmeta), 56.5 (OCH3); 42.8, 37.8, 33.0 (polymer backbone). IR (neat, cm−1): 1500 (m), 1248 (s), 1174 (m), 1031 (m), 828 (m), 541 (m).ROMPH-PNB–NB(CH2)4 triazole (15b) was synthesized in the same way from polymer 8b (1.7 mmol azide per g). Quantitative yield. 13C CP-MAS NMR (100.61 MHz): δ 160.4 (Cpara–OCH3), 149.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR), 137.4–111.4 (C
CR), 137.4–111.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CR, Cortho, NC–Cipso, Cmeta), 56.8 (OCH3); 42.9, 38.6, 33.8 (polymeric backbone). IR (neat, cm−1): 1601 (m), 1500 (s), 1290 (s), 1251 (vs), 1167 (vs), 1031 (vs), 817 (vs), 534 (s), 450 (s).
CR, Cortho, NC–Cipso, Cmeta), 56.8 (OCH3); 42.9, 38.6, 33.8 (polymeric backbone). IR (neat, cm−1): 1601 (m), 1500 (s), 1290 (s), 1251 (vs), 1167 (vs), 1031 (vs), 817 (vs), 534 (s), 450 (s).
General procedure for the Stille reactions using 9b. Synthesis of 4-methoxy-4′-trifluoromethyl-1,1′-biphenyl
Polymer 9b (0.81 g, 0.82 mmol) was placed in a flask under nitrogen and swollen in 40 mL of toluene. 4-Iodotrifluorobenzene (0.98 mL, 0.65 mmol) and [Pd(PPh3)4] (0.19 mg, 0.016 mmol) were added to the polymer. The mixture was kept at 100 °C for 40 h and then it was cooled down to room temperature. MeOH (100 mL) was added to the mixture and the polymer precipitated. It was filtered, washed with MeOH (3 x 20 mL), air-dried and kept overnight at vacuo at 40 °C. The solvents in the filtrate were distilled to ca. 1 mL, and the resulting liquid residue was treated with activated carbon and filtered through a silica column using CH2Cl2 as eluent. Evaporation of the solvent afforded the coupling product as a solid (119.2 mg, 73% yield). It was characterized by 1H and 19F NMR and the spectra compared to those reported in the literature.24 The amount of residual tin in the product was 0.216 mg Sn per g. The yields and conditions of other experiments are collected in Table 5.Results and dicussion
Synthesis of the ROMP and ROMPH ω-bromoalkyl polynorbornenes
Bromoalkylnorbornenes 1a and 1b were synthesized by the Diels–Alder reaction of commercially available dicyclopentadiene and allyl bromide or 6-bromo-1-hexene respectively, as described before.7 Either monomer has a potential advantage in the synthesis of polymeric support materials. The synthesis of 1a is less expensive but, on the other hand, the longer alkyl chain in 1b may be of interest to place a functional group away from the polymeric backbone in order to improve reactivity. Both 1a and 1b are a mixture of endo and exo isomers in a ratio endo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo close to 85
exo close to 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, and this mixture was used in the polymerization experiments. The ROM homopolymerization of 1a or 1b and also de ROM copolymerization of either monomer and norbornene were carried out using Grubbs' 2nd generation catalyst 2 (Scheme 1).
15, and this mixture was used in the polymerization experiments. The ROM homopolymerization of 1a or 1b and also de ROM copolymerization of either monomer and norbornene were carried out using Grubbs' 2nd generation catalyst 2 (Scheme 1).
An amount of catalyst 0.02% mol of the functionalized monomer 1 is enough to achieve high polymerization yields in 4 hours (Table 1) and this amount of catalyst and reaction time were used in all the experiments. Similar good yields can also be obtained if the amount of catalyst is reduced to 0.01% mol but the reaction time needs to be extended to 15 h. Lower catalyst loadings (0.004% mol of the amount of monomer 1) decreased the yield of the reaction dramatically (1.5% yield for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 copolymerization of 1a and norbornene in 15 h). CH2Cl2 was the solvent of choice since it gives better yields than toluene (45% yield for an experiment analogous to entry 3, Table 1) or more coordinating solvents such as acetonitrile or THF (about 10% yield in both cases). The polymers obtained are off-white gum-like solids which are not soluble in most organic solvents, so their molecular weight and polydispersity could not be determined. The copolymer composition was determined by quantitative analysis of the bromo content in the polymer.
1 copolymerization of 1a and norbornene in 15 h). CH2Cl2 was the solvent of choice since it gives better yields than toluene (45% yield for an experiment analogous to entry 3, Table 1) or more coordinating solvents such as acetonitrile or THF (about 10% yield in both cases). The polymers obtained are off-white gum-like solids which are not soluble in most organic solvents, so their molecular weight and polydispersity could not be determined. The copolymer composition was determined by quantitative analysis of the bromo content in the polymer.
As it can be observed in Table 1, the composition of the copolymers does not reproduce the monomer feed ratio and the amount of norbornene in the resulting polymer is always higher. A rough determination of the reactivity ratios for these copolymerizations is consistent with this observation and for the copolymerization of NB/1a the results are rNB = 1.58 and r1a = 0.65, which correspond to an almost ideal random copolymer richer in norbornene. The copolymerization of NB/1b shows rNB = 1.78 and r1b = 0.84, and thus a non-ideal random copolymer again richer in norbornene should be expected. By varying the monomer feed ratio, polymers with a functionalization roughly between 1 to 5 mmol of Br per g of polymer can be obtained. The polymers swell in solvents such as THF, dichloromethane and chloroform by a volume factor between 2 and 3, enough to allow the characterization of these polymers by NMR of the viscous samples. Assignments were made with the help of NMR homo- and heteronuclear correlation experiments and previous literature characterization data (see Experimental section).25 The 1H NMR spectra of the homo- and copolymers show signals in the olefinic region for the trans (δ 5.3) and cis (δ 5.2) stereochemistry of the double bonds. Both arrangements in the polymers are closely represented, with trans![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis ratios that range from 70
cis ratios that range from 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 to 40
30 to 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, as it has been observed before for other polymerizations with this type of catalysts.11j,12d The bromoalkyl substituents show characteristic resonances for the bromomethyl group around 3.5–3.2 ppm. A complicated stereochemistry can be anticipated for these polymers and besides the inherent complex tacticity of ROMP polynorbornene,11j,25 different arrangements of the bromoalkyl monomers are expected (head to head, head to tail, tail to tail) which are visible in the 13C NMR spectra of homopolymers 3a and 3b (x/y = 0) (see ESI†).25c Also, the presence of both configurations for C5 (due to the exo/endo configuration in 1) complicate the structural assignments. Thus, no attempts were made at analyzing the fine structure of the spectra in detail. The IR spectra of the solid polymer samples also show characteristic absorptions for the double bond, ν(
60, as it has been observed before for other polymerizations with this type of catalysts.11j,12d The bromoalkyl substituents show characteristic resonances for the bromomethyl group around 3.5–3.2 ppm. A complicated stereochemistry can be anticipated for these polymers and besides the inherent complex tacticity of ROMP polynorbornene,11j,25 different arrangements of the bromoalkyl monomers are expected (head to head, head to tail, tail to tail) which are visible in the 13C NMR spectra of homopolymers 3a and 3b (x/y = 0) (see ESI†).25c Also, the presence of both configurations for C5 (due to the exo/endo configuration in 1) complicate the structural assignments. Thus, no attempts were made at analyzing the fine structure of the spectra in detail. The IR spectra of the solid polymer samples also show characteristic absorptions for the double bond, ν(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) about 970 (trans) and 740 (cis) cm−1,26 and the bromo substituent, ν(C–Br) in the region 640–550 cm−1.
CH) about 970 (trans) and 740 (cis) cm−1,26 and the bromo substituent, ν(C–Br) in the region 640–550 cm−1.
The insolubility of the polymers is an advantage if they are to be used as solid supports but it prevented their molecular weight and polydispersity index (PDI) determination by GPC. We carried out some polymerizations using ethyl vinyl ether as chain transfer agent (CTA) in a feed ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CTA = 50
CTA = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In this way, shorter polymer chains are expected, hopefully soluble enough to perform a GPC analysis, which could give a minimum value for the molecular weight of the polymers obtained in Table 1. Indeed, the polymers prepared in this way were soluble and showed unimodal distributions in GPC although they are quite polydisperse materials (Table 2).
1. In this way, shorter polymer chains are expected, hopefully soluble enough to perform a GPC analysis, which could give a minimum value for the molecular weight of the polymers obtained in Table 1. Indeed, the polymers prepared in this way were soluble and showed unimodal distributions in GPC although they are quite polydisperse materials (Table 2).
The molecular weights are larger for the copolymers than for the homopolymers and also for the polymerizations involving 1b. It is reasonable to assume that the insoluble polymers obtained without the use of a CTA, suitable to be used as supports, are larger and also very polydisperse.
It has been shown that the second-generation Grubbs' catalyst is very active and, the rate of propagation being larger than the rate of initiation, leads to non-living polymerizations and secondary metathesis processes that increase the polydispersity of the obtained materials.14c,27 We carried out several experiments using a higher amount of catalyst in order to obtain soluble polymers which could give information about the prevalence of secondary metathesis reactions in the polymerization of 1 with complex 2 and/or other cross-linking pathways. Several homopolymerizations of 1a in a 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 = 100
2 = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio were carried out changing the reaction times (entries 1–4, Table 3). A clear decrease in molecular weight is observed along with an increase in the polydispersity and the trans to cis alkene ratio in the polymer, upon increasing the reaction time. These are indications of secondary metathesis on the polymer chains (chain transfer, depolymerization and backbiting) that do not allow to obtain the well-defined polymers characteristic of a living polymerization.12c,28
1 ratio were carried out changing the reaction times (entries 1–4, Table 3). A clear decrease in molecular weight is observed along with an increase in the polydispersity and the trans to cis alkene ratio in the polymer, upon increasing the reaction time. These are indications of secondary metathesis on the polymer chains (chain transfer, depolymerization and backbiting) that do not allow to obtain the well-defined polymers characteristic of a living polymerization.12c,28
An additional experiment was performed that led to similar conclusions. First, the homopolymerization reaction of 1a in a 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 = 100
2 = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio was carried out and, after 40 min, the reaction mixture was split in two batches. The first one was poured onto methanol and a polymer precipitated which was filtered and kept for further analysis (entry 5A, Table 3). The second batch was stirred for a longer time (4 h total time) and then poured onto methanol where a second polymer sample was obtained (entry 5B, Table 3). Again the change in Mn, PDI and trans to cis alkene ratio upon catalyst exposure shows that secondary metathesis is taking place.27,29,30 The additional polymerization reactions collected in Table 3 show that the NB/1 copolymers are generally more polydisperse than the homopolymers (cf. entries 4, 7 and 10, Table 3). The increase of the monomer
1 ratio was carried out and, after 40 min, the reaction mixture was split in two batches. The first one was poured onto methanol and a polymer precipitated which was filtered and kept for further analysis (entry 5A, Table 3). The second batch was stirred for a longer time (4 h total time) and then poured onto methanol where a second polymer sample was obtained (entry 5B, Table 3). Again the change in Mn, PDI and trans to cis alkene ratio upon catalyst exposure shows that secondary metathesis is taking place.27,29,30 The additional polymerization reactions collected in Table 3 show that the NB/1 copolymers are generally more polydisperse than the homopolymers (cf. entries 4, 7 and 10, Table 3). The increase of the monomer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst ratio leads to polymers with higher molecular weight (cf. entries 4 and 6 or 7 and 8, Table 3). In every polymer synthesized or used for the above mentioned experiments, the –CH2Br 1H NMR region shows exactly the same pattern, and the ratio of the integrals for the alkene:CH2Br signals in the homopolymers is invariably 1
catalyst ratio leads to polymers with higher molecular weight (cf. entries 4 and 6 or 7 and 8, Table 3). In every polymer synthesized or used for the above mentioned experiments, the –CH2Br 1H NMR region shows exactly the same pattern, and the ratio of the integrals for the alkene:CH2Br signals in the homopolymers is invariably 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, showing that the bromo functionality does not change and it is not lost in the polymerization process. Thus, it is unlikely that the bromoalkyl chains are involved in cross-linking processes and the lower solubility of the polymers collected in Table 1, where a larger monomer:catalyst ratio is used, must be mostly determined by their large molecular weight along with the effect of secondary metathesis on the polymer chains.
1, showing that the bromo functionality does not change and it is not lost in the polymerization process. Thus, it is unlikely that the bromoalkyl chains are involved in cross-linking processes and the lower solubility of the polymers collected in Table 1, where a larger monomer:catalyst ratio is used, must be mostly determined by their large molecular weight along with the effect of secondary metathesis on the polymer chains.
Saturated ω-bromoalkyl ROMPH-PNBs (4, Scheme 1) were obtained by hydrogenation of the double bonds of the ROMP-PNBs 3. The reduction with excess p-toluenesulfonylhydrazide worked well, and complete disappearance of the olefinic signals were observed in the NMR spectra of the new materials. The polymers were obtained as gum-like off-white solids, insoluble in most organic solvents, which swell in chloroform, CH2Cl2 and THF by a volume swelling factor between 1.2–1.4 for 4a and 1.3–1.9 for 4b. Thus, the extent of swelling of these materials is small, certainly smaller than that observed for polymers 3, but enough to allow the NMR characterization of the viscous samples. Resonances for the bromomethyl group in the NMR spectra (δ 3.5–3.2) and the ν(C–Br) absorption between 640 and 560 cm−1 in IR spectroscopy are characteristic features for 4. The amount of bromo in the ROMPH-PNBs was determined by quantitative analysis and, starting from the different copolymers 3, saturated materials with a functionalization degree between 1 and 5 mmol Br per g can be obtained.
In some reduction experiments, a small loss of bromide (5–15%) was observed in polymers 4 when compared with the calculated amount considering the functionalization of the starting ROMP-PNBs 3, and the slight weight change upon hydrogenation. This decrease in the amount of bromide could be due to a selective loss of bromo-enriched polymeric chains in the reaction workup. Nonetheless, we checked if it was linked to the introduction of an additional undesired functionality in the polymer. An independent experiment treating 1-bromo-3-methylbutane as a model compound with excess p-toluenesulfonylhydrazide, in the same conditions used for the reduction of 3, did not show the loss of the bromo substituent. A HBr elimination in the polymer does not occur since it would result in a methyl generated from the hydrogenation of the new terminal double bond formed, which was not present in the polymer, as checked by 1H and 13C NMR spectroscopy. Thus, the reduction process does not introduce any new reactive center in the polymer which remains a saturated scaffold with bromo as the only functional group present.
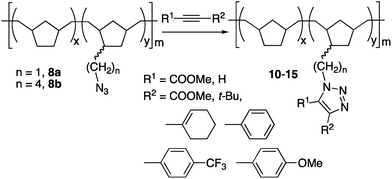
Functionalization of the ROMPH ω-bromoalkyl polynorbornenes 4
The versatility of the ROMPH scaffolds was tested by subjecting 4 to several transformations that can be useful in post-polymerization attachment of a functional group of interest. First, the synthesis of saturated polymers with pendant functionalized alkyl chains was possible by nucleophilic substitution of bromide in polymers 4. Different nucleophiles have been introduced which lead to polymeric materials bearing cyano, azido, thio or ester groups (Scheme 2). Even a bulky nucleophile such as a stannyl group can be introduced. Table 4 collects the polymers prepared by this route. The bromo content of the final polymers was determined to check the efficiency of the substitution, which is very high in most reactions (97–100%), with the exception of the ester substitution (83%, entry 2, Table 4). In the latter case, HBr elimination is also observed as shown by the presence of small olefinic signals in the 1H NMR spectrum of 6a (4.82 and 4.72 ppm). The other nucleophiles collected in Table 4 undergo faster bromo substitution and HBr elimination does not compete.| Entry | 4 (mmol Br per g) | ROMPH-PNB–NB(CH2)nNu, Nu | Residual mmol Br per g | Isolated yield%f, (Br substitution%) |
|---|---|---|---|---|
| a Reaction time 24 h, using the appropriate solvent at reflux.b THF.c Toluene.d DMF.e THF, room temperature.f Calculated considering the residual Br content in the polymer and the weight change upon substitution of Br by Nu (see ESI). | ||||
| 1b | 4a (1.4) | 5a, CN | 0.041 | 98 (97) |
| 2c | 4a (1.4) | 6a, OCOMe | 0.245 | 85 (83) |
| 3c | 4a (1.4) | 7a, SPh | 0.044 | 97 (97) |
| 4d | 4a (1) | 8a, N3 | 0 | 98 (100) |
| 5d | 4b (1.58) | 8b, N3 | 0 | 96 (100) |
| 6e | 4b (1.36) | 9b, SnBu2An | 0 | 92 (100) |
As their parent materials, the polymers are not soluble in common organic solvents. With the exception of 8, they swell in chloroform or THF, enough to allow their characterization by NMR in the viscous mixture. A shift of the methylene protons of the –CH2Nu groups when compared to the –CH2Br parent groups is observed, as well as the presence of the characteristic signals of Nu groups. The IR spectra show the disappearance of the ν(C–Br) absorption along with new absorptions of the Nu groups (see Experimental section). The azido ROMPH polymers 8 could not be characterized by solution NMR and the CP-MAS 13C NMR spectra do not show a distinct –CH2N3 resonance, which overlaps with the broad signals of the polymer. Nonetheless, the IR spectra is very informative and clearly shows the presence of the strong ν(N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) resonances at 2090 cm−1.
N) resonances at 2090 cm−1.
Our former experience with stannylated polymers of different backbones, specially vinylic addition polynorbornenes (VA-PNBs),8,22a,31 led us to introduce a stannyl group on 4b. As shown in Table 4 (entry 6) the substitution works well leading to a polymeric material that can be used as reagent in the Stille reaction. 4b was chosen for this application since polymers with longer pendant alkyl chains have proved to be more reactive.8 Polymer 9b was used in the Stille reaction for the formation of biphenyls in good or moderate yields (Scheme 3 and Table 5). Stille couplings using polymeric materials are generally slower and need longer reaction times than those using monomeric tin derivatives. However, this is counterbalanced by the easy separation of the coupling products from the polymeric halogenated tin byproduct which can be precipitated and filtered in a very convenient way. Using 9b, 4-methoxy-4′-trifluoromethyl-1,1′-biphenyl (entry 2, Table 5) was prepared in a larger scale in a 73% isolated yield with a contamination of only 0.022% weight of tin (see Experimental section). This amount is about 200 times lower than the residual tin found in the preparations using a tributyltin derivative and a more cumbersome work up.31b This reaction shows that ROMPH-PNBs are robust enough to be used in catalysis at high temperature (100 °C in this case). Their performance in this reaction is comparable to the vinylic addition polynorbornenes (VA-PNBs) we reported before.8,31b
| Entry | ArI | t (h) | R-An, Yield (%)b |
|---|---|---|---|
| a Reaction conditions: toluene, 100 °C, 0.1 g 9b (0.1 mmol SnBu2An), 0.08 mmol ArI, [Pd(PPh3)4] as catalyst (2.5% mol).b Crude yield determined by integration of 1H or 19F NMR signals. | |||
| 1 | C6H5I | 20 | C6H5-p-C6H4OMe, 55 |
| 2 | p-CF3C6H4I | 40 | p-CF3C6H4-p-C6H4OMe, 81 |
| 3 | p-FC6H4 I | 40 | p-FC6H4-p-C6H4OMe, 74 |
| 4 | o-MeC6H4I | 40 | o-MeC6H4-p-C6H4OMe, 38 |
| 5 | p-MeC6H4I | 40 | p-MeC6H4-p-C6H4OMe, 62 |
A saturated scaffold is very attractive for a polymeric support in Pd-catalyzed reactions. The parent ROMP polymers, with an unsaturated backbone, are less convenient in these reactions, since alkene insertion into Pd–C bonds is a well-known process that can alter the nature of the ROMP skeleton. This was clearly shown when the soluble copolymer 3a (x/y = 1.11, entry 8, Table 3) was mixed with [PdBrPf(NCMe)2] (Pf = C6F5), a model complex analogous to the palladium aryl species formed in many Pd-catalyzed C–C coupling reactions.32 We have previously shown that alkenes insert into the Pd–C bond of this complex, and the formation of the new C–Pf bond can be clearly seen by 19F NMR where the Fortho signal of the Pf group undergoes a strong shift from around −120 ppm (Pd–Pf) to −140 ppm (C–Pf).33 As can be seen in Fig. 2 the ROMP copolymer reacts with the palladium aryl complex and the pentafluorophenyl group (Pf) is incorporated to the polymeric backbone.
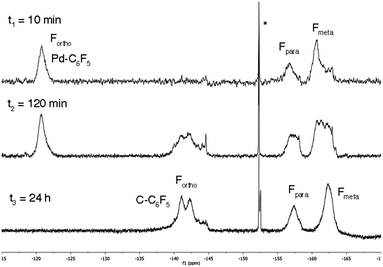 | ||
| Fig. 2 19F NMR spectra for the reaction of [PdBr(C6F5)(NCMe)2] with ROMP copolymer 3a (x/y = 1.1) in CDCl3 at different times. *BF4− impurity from the synthesis of the complex. | ||
The alkyne–azide cycloaddition reaction is one of the most popular and versatile click reactions. The copper-catalyzed version (CuAAC) allows the cycloaddition of a wide variety of substrates,34 and it has been applied to polymer and macromolecule functionalization,34c,35 including ROMP polynorbornenes.36 Thus, we checked the CuAAC reactions on the azido polymers 8 since this route of functionalization opens up a way to anchor a large variety on groups through a 1,2,3-triazole link by selecting the appropriate alkyne (Scheme 4). We checked the reaction with alkynes with different substituents from the diester dimethyl acetylenedicarboxylate, which does not need copper catalysis to form the triazole, to enynes (Table 6). In all cases the triazole is formed and this is shown by the disappearance of the strong azide absorption in the IR spectra (2090 cm−1) and the appearance of characteristic bands for the substituents of the alkyne. All the triazole substituted polymers are insoluble solids and they were characterized by 13C CP-MAS solid state NMR. Resonances for the triazole C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond are shown in the spectra along with other resonances of the specific substituents (see Experimental section).
C bond are shown in the spectra along with other resonances of the specific substituents (see Experimental section).
| Entry | 8, (mmol N3 per g) | R1, R2 | Polymer | Isolated yield (%) |
|---|---|---|---|---|
a The reactions were carried out in THF for 24 h; molar ratio 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alkyne alkyne![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CuI CuI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DIPEA = 1 DIPEA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50.b Without CuI; toluene at reflux.c At room temperature.d At reflux. 50.b Without CuI; toluene at reflux.c At room temperature.d At reflux. |
||||
| 1b | 8a (1) | COOMe, COOMe | 10a | 93 |
| 2c | 8a (1) | H, t-Bu | 11a | 100 |
| 3c | 8a (1) | H, cyclohexenyl | 12a | 97 |
| 4d | 8a (1) | H, C6H5 | 13a | 100 |
| 5c | 8a (1) | H, p-C6H4CF3 | 14a | 96 |
| 6d | 8a (1) | H, p-C6H4OMe | 15a | 100 |
| 7d | 8b (1.7) | H, p-C6H4OMe | 15b | 100 |
Conclusions
New bromosubstituted polymeric materials with a saturated polymeric backbone are available to be used as manifold supports by introducing the appropriate functionality by post-polymerization functionalization. The support is a copolymer of norbornene and a ω-bromoalkyl norbornene which has been synthesized by ring opening metathesis polymerization followed by hydrogenation. The bromo functionality is attached to the saturated polymeric backbone by an alkyl chain tether of different length and nucleophilic substitution of this bromide by different reagents is possible. Among the reactions tested, an azido polymer has been prepared which opens up the possibility to use the alkyne–azide cycloaddition reaction as a tool to link a variety of groups and this has been demonstrated. A stannylated polymer has also been synthesized and used in the Stille coupling to give biphenyls, a Pd-catalyzed reaction that is carried out at high temperature.Thus, a more robust, saturated version of the frequently used ROMP polynorbornene is now available that allows different types of functionalization but does not require the synthesis of the polymer from scratch, i.e. from the prefunctionalized monomer. Not many all-aliphatic polymeric supports are available that can be used by the synthetic chemist, working outside the polymer field. In resemblance to what occurs for the popular and useful functionalized polystyrenes, the ω-bromoalkyl polynorbornenes described here have the potential to be part of the toolbox to devise efficient and green synthetic procedures, specially if the support has to be subjected to reaction conditions not compatible with a more reactive polymer skeleton.
Acknowledgements
Financial support from the Spanish MINECO (DGI, grant CTQ2013-48406 P) and the Junta de Castilla y León (grant VA302U13 and fellowship to RGL co-funded by the European Social Fund) is gratefully acknowledged.References
- (a) A. Puglisi, M. Benaglia and V. Chiroli, Green Chem., 2013, 15, 1790 RSC; (b) A. C. Albéniz and N. Carrera, Eur. J. Inorg. Chem., 2011, 2347 CrossRef PubMed; (c) A. Molnár, Chem. Rev., 2011, 111, 2251 CrossRef PubMed; (d) J. Lu and P. H. Toy, Chem. Rev., 2009, 109, 815 CrossRef CAS PubMed; (e) D. E. Bergbreiter, J. Tian and C. Hongfa, Chem. Rev., 2009, 109, 530 CrossRef CAS PubMed; (f) R. Akiyama and S. Kobayashi, Chem. Rev., 2009, 109, 594 CrossRef CAS PubMed; (g) B. M. L. Dioos, I. F. J. Vankekecom and P. A. Jacobs, Adv. Synth. Catal., 2006, 348, 1413 CrossRef CAS PubMed; (h) C. A. McNamara, M. J. Dixon and M. Bradley, Chem. Rev., 2002, 102, 3275 CrossRef CAS PubMed; (i) N. E. Leadbeater and M. Marco, Chem. Rev., 2002, 102, 3217 CrossRef CAS PubMed; (j) T. J. Dikerson, N. N. Reed and K. D. Janda, Chem. Rev., 2002, 102, 3325 CrossRef PubMed.
- (a) P. J. H. Scott and P. G. Steel, Eur. J. Org. Chem., 2006, 2251 CrossRef CAS PubMed; (b) A. Kirschning, H. Monenschein and R. Wittenberg, Angew. Chem., Int. Ed., 2004, 40, 650 CrossRef; (c) S. Bräse, J. H. Kirchhoff and J. Köbberling, Tetrahedron, 2003, 59, 885 CrossRef; (d) R. E. Sammelson and M. J. Kurth, Chem. Rev., 2001, 101, 137 CrossRef CAS PubMed; (e) B. A. Lorsbach and M. J. Kurth, Chem. Rev., 1999, 99, 1549 CrossRef CAS PubMed.
- T. J. Colacot, W. A. Carole, B. A. Neide and A. Harad, Organometallics, 2008, 27, 5605 CrossRef CAS.
- (a) P. Coupillaud, J. Pinaud, N. Guidolin, J. Vignolle, M. Fèvre, E. Veaudecrenne, D. Mecerreyes and D. Taton, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 4530 CAS; (b) J. Pinaud, J. Vignolle, Y. Gnanou and D. Taton, Macromolecules, 2011, 44, 1900 CrossRef CAS.
- (a) M. Al-Hashimi, C. Hongfa, B. George, H. S. Bazzi and D. E. Bergbreiter, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 3954 CrossRef CAS PubMed; (b) D. E. Bergbreiter, H.-L. Su, H. Koizumi and J. Tian, J. Organomet. Chem., 2011, 696, 1272 CrossRef CAS PubMed.
- X. Wang, Y. Wang, X. Shi, J. Liu, C. Chen and Y. Li, Macromolecules, 2014, 47, 552 CrossRef CAS.
- S. Martínez-Arranz, A. C. Albéniz and P. Espinet, Macromolecules, 2010, 43, 7482 CrossRef.
- S. Martínez-Arranz, N. Carrera, A. C. Albéniz, P. Espinet and A. Vidal-Moya, Adv. Synth. Catal., 2012, 354, 3551 CrossRef PubMed.
- (a) J. A. Molina de la Torre and A. C. Albéniz, ChemCatChem, 2014, 6, 3547 CAS; (b) I. K. Sagamanova, S. Sayalero, S. Martínez-Arranz, A. C. Albéniz and M. A. Pericàs, Catal. Sci. Technol., 2015, 5, 754 RSC.
- M. A. Gauthier, M. I. Gibson and H.-A. Klok, Angew. Chem., Int. Ed., 2009, 48, 48 CrossRef CAS PubMed.
- (a) J. Zhang, M. E. Matta, H. Martínez and M. A. Hillmyer, Macromolecules, 2013, 46, 2535 CrossRef CAS; (b) K.-H. Yoon, K. O. Kim, M. Schaefer and D. Y. Yoon, Polymer, 2012, 53, 2290 CrossRef CAS PubMed; (c) S. Kobayashi, L. M. Pitet and M. A. Hillmyer, J. Am. Chem. Soc., 2011, 133, 5794 CrossRef CAS PubMed; (d) Z. Zhang, K.-Z. Fan, Q.-P. Song and G.-W. Wang, Synth. Commun., 2010, 40, 1052 CrossRef CAS PubMed; (e) M. Lichtenheldt, D. Wang, K. Vehlow, I. Reinhardt, C. Kühnel, U. Decker, S. Blechert and M. R. Buchmeiser, Chem.–Eur. J., 2009, 15, 9451 CrossRef CAS PubMed; (f) J. J. Murphy, J. G. Hamilton and R. M. Paton, Polymer, 2006, 47, 3292 CrossRef CAS PubMed; (g) O. A. Scherman, H. M. Kim and R. H. Grubbs, Macromolecules, 2002, 35, 5366 CrossRef CAS; (h) V. C. Gibson and T. Okada, Macromolecules, 2000, 33, 655 CrossRef CAS; (i) D.-J. Liaw and C.-H. Tsai, J. Mol. Catal. A: Chem., 1999, 147, 23 CrossRef CAS; (j) B. Al-Samak, V. Amir-Ebrahimi, A. G. Carvill, J. G. Hamilton and J. J. Rooney, Polym. Int., 1996, 41, 85 CrossRef CAS; (k) B. H. Sohn, J. A. Gratt, J. K. Lee and R. E. Cohen, J. Appl. Polym. Sci., 1995, 58, 1041 CrossRef CAS PubMed; (l) Z. Wu and R. H. Grubbs, Macromolecules, 1994, 27, 6700 CrossRef CAS; (m) H. G. M. Edwards, D. W. Farwell, A. F. Johnson, I. R. Lewis, N. J. Ward and N. Webb, Macromolecules, 1992, 25, 525 CrossRef CAS.
- (a) C. Dumrath, A. Dumrath, H. Neumann, M. Beller and R. Kadyrov, ChemCatChem, 2014, 6, 3101 CrossRef CAS PubMed; (b) M. T. Showak, A. B. Burns, A. J. Stella and R. A. Register, Macromolecules, 2013, 46, 9288 CrossRef CAS; (c) R. Walker, R. M. Conrad and R. H. Grubbs, Macromolecules, 2009, 42, 599 CrossRef CAS PubMed; (d) Y. Nishihara, S. Izawa, Y. Inoue, Y. Nakayama, T. Shiono and K. Takagi, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3314 CrossRef CAS PubMed; (e) J. P. Bishop and R. A. Register, Macromol. Rapid Commun., 2008, 29, 713 CrossRef CAS PubMed; (f) K. D. Camm, N. Martínez-Castro, Y. Liu, P. Czechura, J. L. Snelgrove and D. E. Fogg, J. Am. Chem. Soc., 2007, 129, 4168 CrossRef CAS PubMed; (g) L.-B. W. Lee and R. A. Register, Macromolecules, 2005, 38, 1216 CrossRef CAS; (h) J. D. Hatjopoulos and R. A. Register, Macromolecules, 2005, 38, 10320 CrossRef CAS; (i) C. M. Dettmer, M. K. Gray, J. M. Torkelson and S. T. Nguyen, Macromolecules, 2004, 37, 5504 CrossRef CAS; (j) N. Cobo, M. A. Esteruelas, F. González, J. Herrero, A. M. Lopez, P. Lucio and M. Oliván, J. Catal., 2004, 223, 319 CrossRef CAS PubMed; (k) S. Hayano, H. Kurakata, Y. Tsunogae, Y. Nakayama, Y. Sato and H. Yasuda, Macromolecules, 2003, 36, 7422 CrossRef CAS; (l) T. Otsuki, K. Goto and Z. Komiya, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 4661 CrossRef CAS.
- Polymers based on the ROMPH polynorbornene are commercialized under the trademark Zeonex® by ZEON corporation: (a) M. Yamazaki, J. Mol. Catal. A: Chem., 2004, 213, 81 CrossRef CAS PubMed; (b) J. C. Mol, J. Mol. Catal. A: Chem., 2004, 213, 39 CrossRef CAS PubMed.
- (a) A. Leitgeb, J. Wappel and C. Slugovc, Polymer, 2010, 51, 2927 CrossRef CAS PubMed; (b) S. Hilf and A. F. M. Kilbinger, Nat. Chem., 2009, 1, 537 CrossRef CAS PubMed; (c) C. W. Bielawski and R. H. Grubbs, Prog. Polym. Sci., 2007, 32, 1 CrossRef CAS PubMed; (d) S. Riegler, C. Slugovc, G. Trimmel and F. Stelzer, Macromol. Symp., 2004, 217, 231 CrossRef CAS PubMed; (e) C. Slugovc, Macromol. Rapid Commun., 2004, 25, 1283 CrossRef CAS PubMed.
- Some examples of ROMP materials with interesting structures and properties: (a) Y. Xia, A. J. Boydstone and R. H. Grubbs, Angew. Chem., Int. Ed., 2011, 50, 5882 CrossRef CAS PubMed; (b) H.-W. Wang, Z.-C. Liu, C.-H. Chen, T.-S. Lim, W. Fann, C.-G. Chao, J.-Y. Yu, S.-L. Lee, C.-H. Chen, S.-L. Huang and T.-Y. Luh, Chem.–Eur. J., 2009, 15, 5719 CrossRef CAS PubMed; (c) S. Sutthasupa, M. Shiotsuki, T. Masuda and F. Sanda, J. Am. Chem. Soc., 2009, 131, 10546 CrossRef CAS PubMed; (d) T. J. Clark, N. J. Robertson, H. A. Kostalik IV, E. B. Lobkovsky, P. F. Mutolo, H. D. Abruña and G. W. Coates, J. Am. Chem. Soc., 2009, 131, 12888 CrossRef CAS PubMed; (e) K. Feng, C. Zuniga, Y.-D. Zhang, D. Kim, S. Barlow, S. R. Marder, J. L. Brédas and M. Weck, Macromolecules, 2009, 42, 6855 CrossRef CAS; (f) F. Jing and M. A. Hillmyer, J. Am. Chem. Soc., 2008, 130, 13826 CrossRef CAS PubMed; (g) A. Meyers, A. Kimyonok and M. Weck, Macromolecules, 2005, 38, 8671 CrossRef CAS.
- (a) G. M. Pawar and M. R. Buchmeiser, Adv. Synth. Catal., 2010, 352, 917 CrossRef CAS PubMed; (b) W. J. Sommer and M. Weck, Adv. Synth. Catal., 2006, 348, 2101 CrossRef CAS PubMed; (c) S. J. Dolman, K. C. Hultzsch, F. Pezet, X. Teng, A. H. Hoveyda and R. R. Schrock, J. Am. Chem. Soc., 2004, 126, 10945 CrossRef CAS PubMed; (d) E. Årstad, A. G. M. Barrett and L. Tedeschi, Tetrahedron Lett., 2003, 44, 2703 CrossRef; (e) M. Mayr, B. Mayr and M. R. Buchmeiser, Angew. Chem., Int. Ed., 2001, 40, 3839 CrossRef CAS.
- (a) N. Naganna and N. Madhavan, Org. Lett., 2013, 15, 5870 CrossRef CAS PubMed; (b) A. M. Harned, M. Zhang, P. Vedantham, S. Mukherjee, R. H. Herpel, D. L. Flynn and P. R. Hanson, Aldrichimica Acta, 2005, 38, 3 CAS; (c) A. G. M. Barrett, B. T. Hopkins and J. Köbberling, Chem. Rev., 2002, 102, 3301 CrossRef CAS PubMed.
- B. S. Lee, S. Mahajan, B. Clapham and K. D. Janda, J. Org. Chem., 2004, 69, 3319 CrossRef CAS PubMed.
- M. A. Gauthier, M. I. Gibson and H.-A. Klok, Angew. Chem., Int. Ed., 2009, 48, 48 CrossRef CAS PubMed.
- (a) D. J. Liaw, C. C. Huang, J.-Y. Ju and J.-T. Liaw, US2005/0182220 A1, 2005; (b) D. J. Liaw and C. C. Huang, Polym. Prepr., 2003, 44, 945 CAS; (c) K. J. Ivin, L.-M. Lam and J. J. Rooney, Macromol. Chem. Phys., 1994, 195, 3245 CrossRef CAS PubMed; (d) S. Hara, Z. Endo and H. Mera, EP0283719A2, 1988.
- D. C. White, Mikrochim. Acta, 1961, 449 CrossRef CAS.
- (a) N. Carrera, A. Salinas-Castillo, A. C. Albéniz, P. Espinet and R. Mallavía, J. Organomet. Chem., 2011, 696, 3316 CrossRef CAS PubMed; (b) N. Carrera, M. H. Pérez-Temprano, A. C. Albéniz, J. A. Casares and P. Espinet, Organometallics, 2009, 28, 3957 CrossRef CAS.
- J. M. G. Cowie, Polymers: Chemistry & Physics of Modern Materials, Chapman & Hall, Cheltenham, 1991 Search PubMed.
- CAS registry number: 10355-12-1. S. E. Denmark, R. C. Smith and S. A. Tymonko, Tetrahedron, 2007, 63, 5730 CrossRef CAS PubMed.
- (a) T. Sunaga, K. J. Ivin, G. E. Hosmeister, J. H. Oskam and R. R. Schrock, Macromolecules, 1994, 27, 4043 CrossRef CAS; (b) J. G. Hamilton, K. J. Ivin and J. J. Rooney, J. Mol. Catal., 1985, 28, 255 CrossRef CAS; (c) K. J. Ivin, G. Lapienis and J. J. Rooney, Polymer, 1980, 21, 436 CrossRef CAS; (d) K. J. Ivin, T. D. Laverty and J. J. Rooney, Makromol. Chem., 1977, 178, 1545 CrossRef CAS PubMed.
- F. Cataldo, Polym. Int., 1994, 34, 49 CrossRef CAS PubMed.
- C. W. Bielawski and R. H. Grubbs, Angew. Chem., Int. Ed., 2000, 39, 2903 CrossRef CAS.
- B. R. Maughon and R. H. Grubbs, Macromolecules, 1996, 29, 5765 CrossRef CAS.
- M. W. Wagaman and R. H. Grubbs, Macromolecules, 1997, 30, 3978 CrossRef CAS.
- A. M. Alb, P. Enohnyaket, J. F. Craymer, T. Eren, E. B. Coughlin and W. F. Reed, Macromolecules, 2007, 40, 444 CrossRef CAS.
- (a) I. Meana, A. C. Albéniz and P. Espinet, Adv. Synth. Catal., 2010, 352, 2887 CrossRef CAS PubMed; (b) N. Carrera, E. Gutierrez, R. Benavente, M. M. Villavieja, A. C. Albéniz and P. Espinet, Chem.–Eur. J., 2008, 14, 10141 CrossRef CAS PubMed.
- (a) A. C. Albéniz and J. A. Casares, Adv. Organomet. Chem., 2014, 62, 1 CrossRef PubMed; (b) P. Espinet, A. C. Albéniz, J. A. Casares and J. M. Martínez-Ilarduya, Coord. Chem. Rev., 2008, 252, 2180 CrossRef CAS PubMed.
- (a) A. C. Albéniz, P. Espinet, B. Martín-Ruiz and D. Milstein, J. Am. Chem. Soc., 2001, 123, 11504 CrossRef; (b) A. C. Albéniz, P. Espinet and Y.-S. Lin, Organometallics, 1997, 16, 4138 CrossRef; (c) A. C. Albéniz, P. Espinet and Y.-S. Lin, Organometallics, 1997, 16, 5964 CrossRef; (d) A. C. Albéniz, P. Espinet and Y.-S. Lin, Organometallics, 1995, 14, 2977 CrossRef; (e) A. C. Albeniz, P. Espinet, C. Foces-Foces and F. H. Cano, Organometallics, 1990, 9, 1079 CrossRef CAS.
- (a) M. Meldal and C. W. Tornøe, Chem. Rev., 2008, 108, 2952 CrossRef CAS PubMed; (b) R. Huisgen, in 1,3-Dipolar Cycloaddition Chemistry, ed. A. Padwa, Wiley, New York, 1984 Search PubMed; (c) R. Huisgen, Proc. Chem. Soc., London, 1961, 357 Search PubMed.
- (a) J. Romulus, J. T. Henssler and M. Weck, Macromolecules, 2014, 47, 5437 CrossRef CAS; (b) P. Golas and K. Matyjaszewski, Chem. Soc. Rev., 2010, 39, 1338 RSC; (c) U. Mansfeld, C. Pietsch, R. Hoogenboom, C. R. Becer and U. S. Schubert, Polym. Chem., 2010, 1, 1560 RSC; (d) R. K. Iha, K. L. Wooley, A. M. Nyström, D. J. Burke, M. J. Kade and C. J. Hawker, Chem. Rev., 2009, 109, 5620 CrossRef CAS PubMed; (e) W. H. Binder and R. Sachsenhofer, Macromol. Rapid Commun., 2007, 28, 15 CrossRef CAS PubMed; (f) V. Ladmiral, G. Mantovani, G. J. Clarkson, S. Cauet, J. L. Irwin and D. M. Haddleton, J. Am. Chem. Soc., 2006, 128, 4823 CrossRef CAS PubMed; (g) M. J. Joralemon, R. K. O'Reilly, C. J. Hawker and K. L. Wooley, J. Am. Chem. Soc., 2005, 127, 16892 CrossRef CAS PubMed.
- (a) M. Schaefer, N. Hanik and A. F. M. Kilbinger, Macromolecules, 2012, 45, 6807 CrossRef CAS; (b) S. K. Yang and M. Weck, Macromolecules, 2008, 41, 346 CrossRef CAS; (c) S. Hilf, N. Hanik and A. F. M. Kilbinger, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 2913 CrossRef CAS PubMed; (d) W. H. Binder and C. Kluger, Macromolecules, 2004, 37, 9321 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional experimental data and NMR and IR spectra as well as SEM images of the new polymers. See DOI: 10.1039/c5ra15187b |
| This journal is © The Royal Society of Chemistry 2015 |