MoS2–graphene nanosheet–CNT hybrids with excellent electrochemical performances for lithium-ion batteries†
Abstract
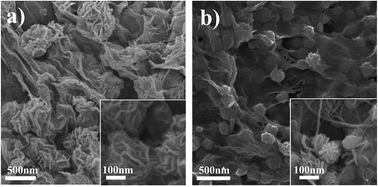
A flower-like MoS2–graphene nanosheet–CNT (MoS2–GNS–CNT) nanocomposite is successfully prepared by a facile hydrothermal process for fast lithium storage. The introduction of a graphene backbone and CNTs prevent the growth of MoS2, resulting in the formation of few layered nano-MoS2 (about 5–10 nm). The CNTs are intimately embedded in the composites to form highly conductive 3D networks, which serve as a highly conductive substrate leading to the high-rate performance. In addition, CNTs in the unique hybrid nanostructure prevent the restacking of GNS. The MoS2–GNS–CNT composite exhibits superior rate capability (300 mA h g−1 at 20 A g−1) and ultralong cyclability (728 mA h g−1 at 5 A g−1 after 1000 cycles). We believe that our strategy could be broadly applicable for the preparation of other transition metal dichalcogenide (TMD) materials with great promise for various applications.

 Please wait while we load your content...
Please wait while we load your content...