Catalytic low-temperature combustion of dichloromethane over V–Ni/TiO2 catalyst
Abstract
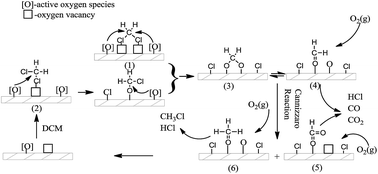
Vanadium–nickel mixed oxides supported on TiO2 (anatase) were prepared by wet impregnation using ammonium metavanadate and nickel nitrate aqueous solution. The performance of as-prepared samples in catalytic dichloromethane (DCM) combustion was investigated, and their physicochemical properties were characterized in detail by X-ray diffraction, N2 physisorption, H2 temperature-programmed reduction, NH3 temperature-programmed desorption, and Raman spectroscopy analyses. Results showed DCM combustion activity over V–Ni/TiO2 catalyst was superior to that of V2O5/TiO2 and NiO/TiO2 catalysts. DCM could be completely converted into CO2, HCl, and a little amount of CO over Ni–V/TiO2 catalyst at 350 °C, the toxic by-products, such as CH3Cl, aldehydes and phosgene could not be observed by online IR spectroscopy. The high catalytic activity, selectivity, and stability of V–Ni/TiO2 catalyst could be due to the good oxidative dehydrogenation ability (ODH), the good reducibility of active oxygen species, and suitable strength of Lewis acidic sites upon introduction of nickel oxide.

 Please wait while we load your content...
Please wait while we load your content...