Investigation of quinoline-4-carboxylic acid as a highly potent scaffold for the development of alkaline phosphatase inhibitors: synthesis, SAR analysis and molecular modelling studies†
Abstract
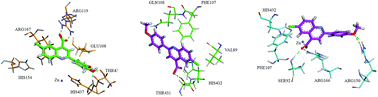
The role played by organic chemistry in the pharmaceutical industry continues to be one of the main drives in the drug discovery process. More than ever, the industry demands from organic chemists the development of small molecules, which could be a rich source of biological potential. In this context, a diverse range of quinoline-4-carboxylic acid derivatives has been synthesized and evaluated as potent inhibitors of alkaline phosphatases. The structural build-up of the synthesized compounds was based on the spectro-analytical data. Most of the tested compounds showed remarkable inhibition of human tissue-nonspecific alkaline phosphatase (h-TNAP), tissue specific human intestinal alkaline phosphatase (h-IAP) and human placental alkaline phosphatase (h-PLAP). Among them, 3j was identified as a potent inhibitor of h-TNAP with an IC50 value of 22 ± 1 nM, whereas, 3e emerged as a lead candidate against h-IAP and h-PLAP with IC50 values of 34 ± 10 and 82 ± 10 nM, respectively. 3a was a potent inhibitor of human germ cell alkaline phosphatase (h-GCAP) with an IC50 value of 150 ± 70 nM. The putative binding sites of the most potent inhibitors were inferred from molecular docking simulations using homology models based on the h-PLAP structure.

 Please wait while we load your content...
Please wait while we load your content...