Inorganic microcapsules mineralized at the interface of water droplets in ethanol solution and their application as drug carriers†
Abstract
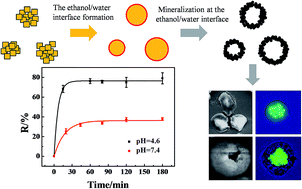
This paper reported a crystallization – dissolution – interface mineralization (CDIM) method on synthesizing calcium carbonate (CaC) and calcium phosphate (CaP) inorganic microcapsules with good biocompatibility and good pH sensitivity. The method is based on mineralization at the ethanol/water interface. The microcapsules were formed in a few seconds and did not need post treatment for removing the templates. The diameters of the microcapsules can be controlled by the size of the crystal clusters regulated by stirring time. Carboxyfluorescein (CF) molecules as model drugs were encapsulated inside the capsules after coating with FeIII–polyphenol tannic acid (TA) films. The pH sensitive carboxyfluorescein molecule releasing behavior was investigated. The lower pH caused faster and thorough release of CF. The CDIM method can be applied for fabricating other inorganic microcapsules, which holds great potential for drug delivery.

 Please wait while we load your content...
Please wait while we load your content...