Green extraction methods and environmental applications of carotenoids-a review
Abstract
This review covers and discusses various aspects of carotenoids including their chemistry, classification, biosynthesis, extraction methods (conventional and non-conventional), analytical techniques and biological roles in living beings. Carotenoids play a very crucial role in human health through foods, cosmetics, nutraceuticals and pharmaceuticals. Among carotenoids, lycopene acts as best antioxidant. Various extraction methods have been employed for extraction of carotenoids: solvent extraction, soxhlet extraction, centrifugation and non-conventional methods of extraction such as ultrasound-assisted, microwave-assisted, enzymatic and the innovative technique supercritical carbondioxide (SC-CO2) extraction. The green and environmentally friendly technique for extraction of carotenoids is SC-CO2 extraction which extracts pure compound in high yield without the use of harmful organic solvents, it operates at lower temperature so it is useful for extraction of thermolabile compounds. This technique uses SC-CO2 as green solvent and other solvents as modifiers which are generally recognized as safe (GRAS) solvents. Green technology is the need of present time in order to keep environment healthy, pollution free and sustainable for coming generation. Present review includes several analytical techniques used to identify and quantify carotenoids are: thin layer chromatography (TLC), high performance thin layer chromatography (HPTLC), high performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), nuclear magnetic resonance (NMR), Fourier transform infrared spectroscopy (FTIR), ultra performance liquid chromatography-tandem mass spectrometer (UPLC-MS), UV-Vis (Ultraviolet-Visible) spectrophotometry; out of these, NMR and FTIR have been explored the least for carotenoid analysis.
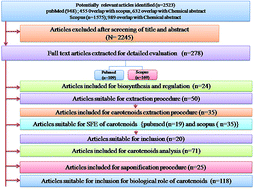

 Please wait while we load your content...
Please wait while we load your content...