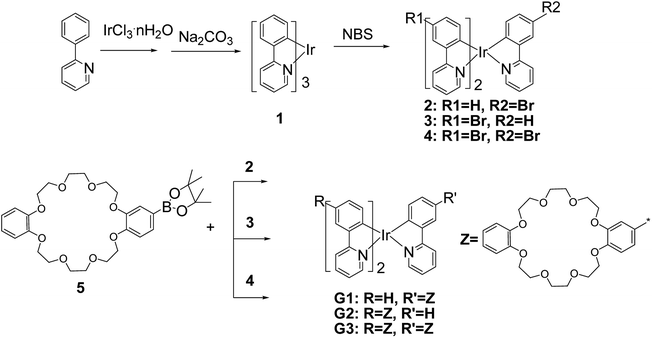
A novel cyclometalated Iridium(III) complex containing dibenzo-24-crown-8: synthesis, luminescence and application in highly efficient green phosphorescent OLEDs†
Aihui Liang*,
Gui Huang,
Shuiliang Chen and
Haoqing Hou
Key Laboratory of Functional Small Organic Molecules, Ministry of Education and College of Chemistry and Chemical Engineering, Jiangxi Normal University, Nanchang 330022, P. R. China. E-mail: lah14god@163.com
First published on 29th May 2015
Abstract
Several new iridium complexes with the dibenzo-24-crown-8 substituted 2-penylpyridine (ppy) ligand have been synthesized and characterized. These iridium complexes emit green light with a high photoluminescence quantum yield ranging from 0.56 to 0.63 and exhibit good thermal properties. The complexes were incorporated into phosphorescent polymer light-emitting devices (PLEDs) using soluble poly(N-vinylcarbazole) (PVK) as the host and the resultant iridium complexes as dopants. High-efficiency green electrophosphorescence was observed, with peak emission at about 520 nm. Among these devices, the PLEDs with G3 as the dopant displayed the best EL performances. An external quantum efficiency of 10.92% and a luminous efficiency of 29.6 cd A−1 were obtained at 537 cd m−2.
Introduction
Organic light-emitting diodes (OLEDs) have been recognized as one of the most promising candidates for flat-panel displays and solid-state lighting because of their high efficiency, low fabrication cost, ease of fabricating large area devices, and wide range of emission colors.1 In order to obtain highly efficient OLEDs, phosphorescent complexes have been extensively developed as emissive materials, since they can harvest both singlet and triplet excitons, which can potentially ensure nearly 100% internal quantum efficiency.2 Among the various phosphorescent emitters, cyclometalated iridium complexes are of paramount importance in the field because of their short lifetime in excited states (∼μs) and high phosphorescent efficiencies for highly efficient OLEDs.3 The most widely used homoleptic green emitting iridium complex, fac-tris[2-phenylpyridinato]Ir(III) [fac-Ir(ppy)3], derivatives have shown a number of advantages such as ease of tuning emission energy by functionalizing the ligand of ppy with electron donating and electron withdrawing substituents and high phosphorescent quantum efficiency at room temperature. In 1985, King et al. first synthesized triply coordinated neutral fac-Ir(ppy)3 and investigated its photophysical and photochemical properties.4 Ir(ppy)3 in degassed toluene shows very strong phosphorescence (λmax = 514 nm, photoluminescence quantum yield ΦPL = 0.4) emitted from the predominantly 3MLCT (metal-to-ligand charge transfer) excited state at room temperature. Afterward, Baldo et al. made an OLED with fac-Ir(ppy)3 as a green phosphorescent dopant. The OLED device can utilize all of the electrogenerated singlet and triplet excitons, leading to ca. 100% internal quantum efficiency.5 The structural modifications of ppy ligand with various substituents have been carried out for the fine tuning of emission colors in the green region and to improve the device efficiencies for the lighting and display applications. In 2004, Jung et al. attached the methyl groups as substituents on the ppy ligand of Ir(ppy)3 to prepare a series of fac-[Ir(dmppy)3] complexes [Hdmppy = n-methyl-2-(n′-methylphenyl)pyridine]. They found that the methyl groups at the meta (Ph) and para (Py) positions to the Ir metal atom have a great influence on absorption, emission, redox potentials and electroluminescence.6 Later on, their group also synthesized the bulky trimethylsilyl substituted iridium(III) complex, fac-tris[4-methyl-2-(4′trimethylsilylphenyl)pyridine]iridium(III) [Ir(msippy)3]. The device with Ir(msippy)3 as dopant and (4,4′-N,N′-dicarbazole)biphenyl (CBP) as host exhibited the maximum external quantum efficiency (EQE) of 25.6%.7While vacuum deposition is often used to OLEDs based on iridium complexes, the solution process is much more desirable, especially for low cost, large-area displays and lighting source.8 Therefore, in many cases, a doping technique must be employed in the fabrication of high-performance solution-processable polymer light-emitting devices (PLEDs). Dispersion of the iridium complex in a polymer host matrix not only separates the phosphor and avoids self-quenching, but also contributes to charge transport. In 2003, Zhu et al. introduced tert-butyl in ppy ligand to achieve fac-tris[2-(4-tert-butylphenyl)pyridinato]iridium with peak emission at about 495 and 515 nm, with which the PLEDs exhibited an EQE of 4.4% and a luminous efficiency (LE) of 10 cd A−1.9
In order to achieve high-efficiency solution-processable green phosphorescent PLEDs, in this contribution, the iridium complexes, whose ppy ligand was modified by group of dibenzo-24-crown-8, were synthesized and emitted green light with a ΦPL ranging from 0.56 to 0.63. By using the novel homoleptic iridium complexes as dopant and poly(N-vinylcarbazole) (PVK) as host, high-efficiecy PLEDs (the best EL performance with a maximum EQE of 10.92% and a maximum LE of 29.6 cd A−1 based on G3) were obtained.
Results and discussion
Synthesis
The synthesis of iridium complexes G1, G2 and G3 was presented in Scheme 1. Compound 2, 3 and 4 were synthesized according to the reported procedure.10 The iridium complex G1 was obtained by Suzuki cross-coupling reaction of compound 2 with 1.2 equiv. of 4-(boron acid pinacol ester)-dibenzo-24-crown-8 (5) in the presence of catalytic amounts of Pd(PPh3)4 and excess K2CO3 in a toluene–ethanol–water solvent system at 90 °C led to a 64% yield. The iridium complexes G2 and G3 were acquired in yields of 61% and 67% with 3 and 4 equivalents of compound 5, respectively, according to the procedure of G1. It is noteworthy that additional 10 mL tetrahydrofuran (THF) was added into the reaction system when the iridium complex G3 was synthesized, as compound 4 is difficult to dissolve in toluene.Optical analysis
The ultraviolet-visible (UV) absorption and photoluminescence (PL) spectra of G1, G2 and G3 in CH2Cl2 and neat films are shown in Fig. 1, and the relevant data are presented in Table 1. The absorption spectra of these complexes in solution are all very similar. The bands below 340 nm are assigned to the spin-allowed 1π–π* ligand-centered transition. The weak absorption shoulders in the range 340–500 nm can be assigned to singlet and triplet MLCT transitions and 3π–π* transitions. Nearly identical PL profiles are observed for G1, G2 and G3 in CH2Cl2 solution and neat films. All the iridium complexes displayed green emission with a maximal phosphorescent peak at ca. 522 nm in solution and 529 nm in neat films. Compared to the PL emission peaks in solution, these iridium complexes show minor red-shifted emission with ca. 6 nm in neat film. Moreover, the resulting iridium complexes exhibited about 10 nm red-shift in solution relative to that of Ir(ppy)3, which could be ascribed to the introduction of crown units into cyclometalated ligand extended the conjugation length.5,6 Furthermore, The PL quantum yields (ΦPL) were obtained relative to [Ir(ppy)3] (ΦPL = 0.4)11 and increase in the order G3 > G2 > G1. The observed high ΦPL value for G3 can be attributed partially to the reduction of intermolecular interactions by the steric effect of bulky 24-crown-8 units, which would result in the concentration quenching and deteriorate the device efficiencies.12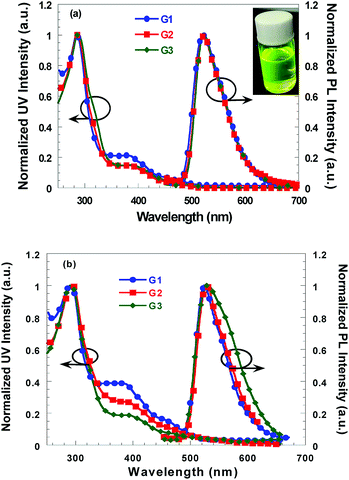 | ||
| Fig. 1 Normalized UV absorption and PL spectra of G1, G2 and G3 in CH2Cl2 (a) and in neat film (b) at room temperature. | ||
Thermal property
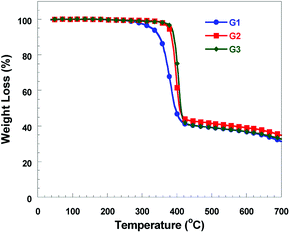
The thermal stability of iridium complexes was evaluated by thermogravimetric analysis (TGA) under a stream of N2 with a scanning rate of 20 °C min−1. Their TGA curves are shown in Fig. 2 and degradation temperatures (Td) for 5% weight loss are listed in Table 1. The recorded Td level is 327 °C for G1, 377 °C for G2 and 384 °C for G3, respectively. The data show that these functionalized iridium complexes possess good thermal properties and the Td is in the order of G3 > G2 > G1, which indicates that dibenzo-24-crown-8 groups was introduced into cyclometalated ligand of the iridium complex can efficiently improve the thermal properties.Electrochemical property
The electrochemical behavior of the three cyclometalated iridium complexes was measured by cyclic voltammetry using ferrocene as the internal standard and their data are listed in Table 2. Reversible oxidation peaks presented at 0.93 ± 0.15 V for these iridium complexes. On the basis of these onset oxidation potentials (Eox) and the formula of EHOMO = −(Eox + 4.38) eV, the highest occupied molecular orbital energy levels (EHOMO) were calculated to be −5.16 eV for G1, −5.18 eV for G2 and −5.46 eV for G3, respectively. As the reduction peak was not displayed, the lowest unoccupied molecular orbital energy levels (ELUMO) had to be calculated based on the equation of ELUMO = EHOMO + Eg, in which energy band gap (Eg) was estimated from the UV-vis absorption threshold.13 As a result, the ELUMO was −2.49 eV for G1, −2.52 eV for G2 and −2.80 eV for G3 were obtained. Apparently, the pendant dibenzo-24-crown-8 groups have an important influence on the HOMO and LUMO energy levels of their iridium complexes. Furthermore, the functionalized iridium complexes with increasing dibenzo-24-crown-8 groups in the cyclometalated ligand exhibited a decreasing ELUMO and ELUMO value due to the effect of the carrier-transporting groups.| Complex | Eox (V) | Ered (V) | Eg (eV) | EHOMO (eV) | ELUMO (eV) |
|---|---|---|---|---|---|
| G1 | 0.78 | −1.89 | 2.67 | −5.16 | −2.49 |
| G2 | 0.80 | −1.86 | 2.66 | −5.18 | −2.52 |
| G3 | 1.08 | −1.58 | 2.66 | −5.46 | −2.80 |
Electroluminescent property
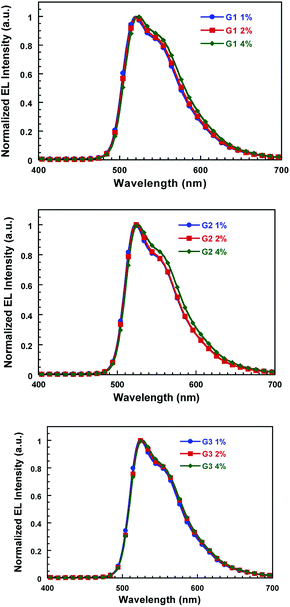
To investigate the influence of dibenzo-24-crown-8 group on electroluminescence (EL) properties of its iridium complex, the devices with single emissive layer were fabricated with a structure of ITO/PEDOT:PSS (40 nm)/Gn (n = 1, 2, 3) + PVK-PBD (90 nm)/CsF (1.5 nm)/Al (100 nm). The emitting layer consists of G1, G2 or G3 dopant and polymeric host matrix of PVK-PBD blend, in which the weight concentration of the dopant is 1, 2 and 4 wt%, PBD weight ratio is 30 wt% in the PVK-PBD blend, respectively.Fig. 3 showed the EL spectra of these novel iridium complexes at different dopant concentrations of 1, 2 and 4 wt% at 12 V. All of these EL spectra exhibited a green emission band from 500 to 650 nm, which arose solely from the iridium complexes. Moreover, there is little difference between the EL spectra which are identical to the corresponding solid-state PL emission as increased the concentrations of the iridium complexes from 1 wt% to 4 wt%.
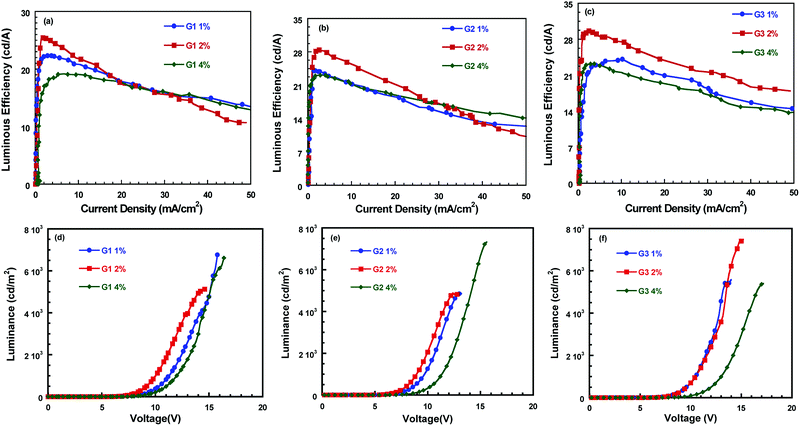
The device performances with these iridium complexes as emissive layer are shown in Fig. 4 and the relevant data are listed in Table 3. As for these devices, when the content of dopant iridium complexes varies from 1 wt% to 2 wt%, the luminous efficiencies (LEs) enhanced with increasing the concentration of iridium complexes. Nevertheless, the LEs decreased as the concentration of the iridium complexes in the emissive layer further increased from 2 wt% to 4 wt%. The efficiency data for the devices with the best performances are shown as follows: for G1 doped device: the turn-on voltage (Von) = 5.2 V and the maximum LE (LEmax) = 25.6 cd A−1 at a current density of 3.15 mA cm−2; for G2 doped device: Von = 4.4 V and LEmax = 28.4 cd A−1 at a current density of 2.6 mA cm−2; for G3 doped device: Von = 5.2 V and LEmax = 29.6 cd A−1 at a current density of 2.75 mA cm−2. The EL performances for these doping devices are in the order of G1 < G2 < G3. This indicated that the more dibenzo-24-crown-8 groups pended on the cyclometalated ligand, the iridium complex displayed better electroluminescent performances, which probably because the encapsulation of the iridium center by the dibenzo-24-crown-8 unit.
| Device | Vona (V) | Vb (V) | Jb (mA cm−2) | QEb (%) | Lmax (cd m−2) | LEmax (cd A−1) | |
|---|---|---|---|---|---|---|---|
| a The turn-on voltage at which luminescence reach 1 cd m−2.b Device data at maximum LE. | |||||||
| G1 | 1% | 5.8 | 10.8 | 1.11 | 9.5 | 6759 | 22.3 |
| 2% | 5.2 | 8.6 | 3.15 | 8.3 | 5123 | 25.6 | |
| 4% | 6.2 | 13 | 9.3 | 7.1 | 6613 | 19.1 | |
| G2 | 1% | 4.4 | 8.8 | 1.9 | 8.8 | 4816 | 24.1 |
| 2% | 4.8 | 8.6 | 2.6 | 10.45 | 4856 | 28.4 | |
| 4% | 5.8 | 11 | 3.2 | 8.47 | 7334 | 23.1 | |
| G3 | 1% | 5.4 | 11.8 | 10.2 | 8.92 | 5582 | 24.0 |
| 2% | 5.2 | 10.2 | 2.75 | 10.92 | 7485 | 29.6 | |
| 4% | 6.6 | 12.4 | 2.8 | 8.58 | 5404 | 23.3 | |
Conclusions
In summary, three novel dibenzo-24-crown-8 unit-based iridium complexes for single emissive layer PLEDs were synthesized and characterized in this study. These iridium complexes emitted green light and exhibited high photoluminescence quantum yield ranging from 0.56 to 0.63 and good thermal properties. The PLEDs based on these iridium complexes as dopant and PVK-PBD as host fabricated by spin-coating technique exhibited promising EL performances. Among these, the device with G3 as dopant displayed the best device's performances with a maximum EQE of 10.92% and a maximum LE of 29.6 cd A−1.Acknowledgements
This work was supported by the Foundation of the Natural Science Foundation of China (no. 51403088).Notes and references
- (a) C. W. Tang and S. A. VanSlyke, Appl. Phys. Lett., 1987, 51, 913 CrossRef CAS PubMed; (b) J. H. Burroughes, D. D. C. Bradley, A. R. Brown, R. N. Marks, K. Mackay, R. H. Friend, P. L. Burns and A. B. Holmes, Nature, 1990, 347, 539 CrossRef CAS PubMed; (c) D. Braun and A. J. Heeger, Appl. Phys. Lett., 1991, 58, 1982 CrossRef CAS PubMed; (d) H. Sasabe, N. Toyota, H. Nakanishi, T. Ishizaka, Y. J. Pu and J. Kido, Adv. Mater., 2012, 24, 3212 CrossRef CAS PubMed; (e) J. Kido, M. Kimura and K. Nagai, Science, 1995, 267, 1332 CrossRef CAS PubMed; (f) S. Thiery, D. Tondelier, C. Declairieux, B. Geffroy, O. Jeannin, R. Métivier, J. Rault-Berthelot and C. Poriel, J. Phys. Chem. C., 2015, 119, 5790 CrossRef CAS; (g) H. Sun, Q. Guo, D. Yang, Y. Chen, J. Chen and D. Ma, ACS Photonics, 2015, 2, 271 CrossRef CAS.
- (a) Y. G. Ma, H. Y. Zhang, J. C. Shen and C. M. Che, Synth. Met., 1998, 94, 245 CrossRef CAS; (b) M. A. Baldo, D. F. O'Brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson and S. R. Forrest, Nature, 1998, 395, 151 CrossRef CAS; (c) S. Gong, C. Yang and J. Qin, Chem. Soc. Rev., 2012, 41, 4797 RSC; (d) M. Zhu, J. Zou, X. He, C. Yang, H. Wu, C. Zhong, J. Qin and Y. Cao, Chem. Mater., 2011, 24, 174 CrossRef; (e) X. Yang, X. Xu, J. Zhao, J.-s. Dang, Z. Huang, X. Yan, G. Zhou and D. Wang, Inorg. Chem., 2014, 53, 12986 CrossRef CAS PubMed.
- (a) C. L. Ho, W. Y. Wong, G. J. Zhou, B. Yao, Z. Xie and L. Wang, Adv. Funct. Mater., 2007, 17, 2925 CrossRef CAS PubMed; (b) A. B. Tamayo, B. D. Alleyne, P. I. Djurovich, S. Lamansky, I. Tsyba, N. N. Ho, R. Bau and M. E. Thompson, J. Am. Chem. Soc., 2003, 125, 7377 CrossRef CAS PubMed; (c) W.-Y. Wong, G.-J. Zhou, X.-M. Yu, H.-S. Kwok and B.-Z. Tang, Adv. Funct. Mater., 2006, 16, 838 CrossRef CAS PubMed; (d) Y. Miao, X. Du, H. Wang, H. Liu, H. Jia, B. Xu, Y. Hao, X. Liu, W. Li and W. Huang, RSC Adv., 2015, 5, 4261 RSC; (e) A. Kapturkiewicz and G. Angulo, Dalton Trans., 2003, 3907 RSC.
- K. A. King, P. J. Spellane and R. J. Watts, J. Am. Chem. Soc., 1985, 107, 1431 CrossRef CAS.
- M. A. Baldo, S. Lamansky, P. E. Burrows, M. E. Thompson and S. R. Forrest, Appl. Phys. Lett., 1999, 75, 4 CrossRef CAS PubMed.
- S. Jung, Y. Kang, H.-S. Kim, Y.-H. Kim, C.-L. Lee, J.-J. Kim, S.-K. Lee and S.-K. Kwon, Eur. J. Inorg. Chem., 2004, 2004, 3415 CrossRef PubMed.
- S. O. Jung, Y.-H. Kim, H.-S. Kim, S.-K. Kwon and H.-Y. Oh, Mol. Cryst. Liq. Cryst., 2006, 444, 95 CrossRef CAS PubMed.
- (a) G. Zhou, Y. He, B. Yao, J. Dang, W.-Y. Wong, Z. Xie, X. Zhao and L. Wang, Chem.–Asian J., 2010, 5, 2405 CrossRef CAS PubMed; (b) Q. Zhao, S.-J. Liu and W. Huang, Macromol. Rapid Commun., 2010, 31, 794 CrossRef CAS PubMed; (c) C. Zhong, C. Duan, F. Huang, H. Wu and Y. Cao, Chem. Mater., 2010, 23, 326 CrossRef; (d) H. Wu, G. Zhou, J. Zou, C.-L. Ho, W.-Y. Wong, W. Yang, J. Peng and Y. Cao, Adv. Mater., 2009, 21, 4181 CrossRef CAS PubMed.
- W. Zhu, C. Liu, L. Su, W. Yang, M. Yuan and Y. Cao, J. Mater. Chem., 2003, 13, 50 RSC.
- P. Stossel, H. Spreitzer and H. Becker, US Pat., 20040138455A1, 2004.
- A. B. Tamayo, B. D. Alleyne, P. I. Djurovich, S. Lamansky, I. Tsyba, N. N. Ho, R. Bau and M. E. Thompson, J. Am. Chem. Soc., 2003, 125, 7377 CrossRef CAS PubMed.
- H. Z. Xie, M. W. Liu, O. Y. Wang, X. H. Zhang, C. S. Lee, L. S. Hung, S. T. Lee, P. F. Teng, H. L. Kwong, H. Zheng and C. M. Che, Adv. Mater., 2001, 13, 1245 CrossRef CAS.
- Z. Si, J. Li, B. Li, Z. Hong, S. Liu and W. Li, J. Phys. Chem. C, 2008, 112, 3920 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed information about synthesis, characterization, and device fabrication conditions. See DOI: 10.1039/c5ra07051a |
| This journal is © The Royal Society of Chemistry 2015 |