Highly moisture-resistant epoxy composites: an approach based on liquid nano-reinforcement containing well-dispersed activated montmorillonite
Abstract
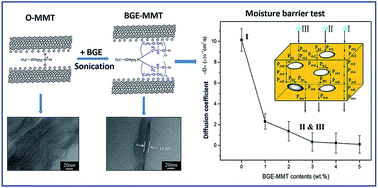
The effects of butyl glycidyl ether (BGE) activated montmorillonites (BGE-MMTs) on moisture-resistant characteristics of epoxy-based composites were evaluated. The activated MMTs were prepared by intercalating BGE into the inter-layer surfaces of octadecyl ammonium modified MMTs (O-MMTs) under ultrasonication, and in the form of liquid nano-reinforcement. It showed advantages of low viscosity, excellent dispersibility and high chemical reactivity in the epoxy matrix. The enhancements in tensile and flexural properties of BGE-MMTs/epoxy composites confirmed the well dispersion of BGE-MMTs in epoxy matrix and the strong interfacial adhesion between the two components. More importantly, the well-dispersed BGE-MMTs in epoxy matrix led to significant enhancement in the moisture-barrier properties of epoxy composites. In comparison with that of neat epoxy, the moisture diffusion coefficient of BGE-MMTs/epoxy composites significantly decreased from 10.1 × 10−6 to 0.3 × 10−6 cm2 s−1. The enhancement in moisture-barrier properties was ascribed to the exfoliated two-dimensional lamellar structure of MMTs extending the effective penetration paths of water molecules into tortuous forms. A model concerning moisture diffusion in BGE-MMTs/epoxy composites was suggested.

 Please wait while we load your content...
Please wait while we load your content...