Synthesis of Pb2O electrocatalyst and its application in the electrochemical reduction of CO2 to HCOOH in various electrolytes
V. S. K. Yadav and
M. K. Purkait*
Department of Chemical Engineering, Indian Institute of Technology, Guwahati-781089, Assam, India. E-mail: mihir@iitg.ernet.in; Fax: +91 361 2582291; Tel: +91 361 2582262
First published on 27th April 2015
Abstract
The reduction of CO2 to products electrochemically (RCPE) using synthesized Pb2O and Co3O4 electrocatalysts in various electrolytes was investigated under ambient conditions. The catalyst (Pb2O) was synthesized electrochemically using an electrodeposition technique. Electrodes were prepared using the synthesized electrocatalysts coated on a graphite plate surface for RCPE. Pb2O/(graphite plate) and Co3O4/(graphite plate) were used as the cathode and anode, respectively, in RCPE. The experiments were conducted at different applied voltages (1.5 to 3.5 V) and time intervals of 5, 10, 15, 20 and 25 min using both carbonates and bicarbonates of sodium and potassium electrolytes separately. Formic acid (HCOOH) was formed for all the applied voltages at different time intervals in the presence of the four electrolytes considered herein. However, higher Faradaic efficiencies were obtained for the bicarbonate based solutions than the carbonates. At 2 V, maximum Faradaic efficiencies of ∼60% and ∼50% for HCOOH were obtained after 10 min of reaction in KHCO3 and NaHCO3 electrolyte solutions, respectively.
1. Introduction
Emission of greenhouse gases in the atmosphere is very critical during combustion of fossil fuels for energy generation. CO2 is one of the primary greenhouse gases causing the global warming effect. A possible solution would be to find a suitable solution for the reduction of this gas back to fuel materials. Thus, reduction of CO2 has become an important topic in the field of electrochemistry. Various technologies such as thermochemical, photochemical, and electrochemical do exist for the reduction of CO2 to various products including methanol, ethanol, formic acid and formaldehyde.1–7 Due to its easy operation, reduction of CO2 to products electrochemically (RCPE) has received great attention among the available techniques.8 In RCPE, electrical energy can be stored in the form of chemical energy, which can be used more widely in transportation applications.9 In the electrochemical process, different products are obtained depending on the material used for the electrodes and electrolytes (aqueous and non-aqueous) and the applied voltage.10–14 Based on the electrocatalyst used, various products like methanol, ethanol, formic acid, formaldehyde, acetic acid, methane and ethylene have been reported.15–18 Hori et al. described that efficiency and product selectivity mainly depended on the electrocatalyst's crystal surfaces and the operating conditions used for RCPE.19 Electrocatalysts like Ag, Sn and Au were able to reduce CO2 to CO, HCOOH and H2 with some other liquid products.15 The reduction of CO2 was also investigated using Pb and Sn electrocatalysts.20–22 The type of electrolytes used in RCPE also plays a major role in product formation. It was reported that products like CO, H2C2O4, HCOOH were formed by using acetonitrile, N,N-dimethyl formamide, and propylene carbonate as electrolytes.23 It has been reported that if liquid products were made with high Faradaic efficiencies from RCPE, it could become a sustainable approach for future liquid fuel production.22Different products were reported from the reduction of CO2 electrochemically. However, conversion of CO2 to formic acid is the best process with respect to technical development and economic viability because of its requirement in paper, pharmaceuticals and pulp industries.24,25
Synthesis of HCOOH by hydrocarbon oxidation or thermochemical process bears adverse environmental effects and expensive.22 It may be envisaged from the above literatures that different products were produced from the reduction of CO2 electrochemically. However, conversion of CO2 to formic acid may be an alternate one but needs detail investigations both on technical development and economic viability. Several studies have been reported towards the reduction of CO2 to HCOOH.24,26 Subramanian et al. studied the RCPE using iridium oxide as anode and lead as cathode towards CO2 reduction.26 Platinum (Pt) was efficiently used as anode for water oxidation reaction.22 Although the reaction is feasible, but multiple products are formed in presence of various catalysts.16 Experimental conditions are also important on the type of product formation using different catalyst.11,27 Finding of an alternate catalyst to reduce CO2 to a single product with high Faradaic efficiency is the ultimate goal of the current research. Co3O4 is abundant, cheap and efficient towards water oxidation reaction which might be better alternate electrocatalyst in place of Pt for RCPE. Use of Co3O4 as anode and Pb2O as cathode towards RCPE is scant. This is focused on the RCPE using cheaper Co3O4 as anodic material. Pb2O powder was prepared using electrodeposition method as it requires room temperature, low process time and cost effective.
The present work is to study the performance of RCPE using synthesized Pb2O as cathode and Co3O4 as anode towards the HCOOH formation in the presence of carbonates and bicarbonates of sodium and potassium electrolytes. A 2-electrode system was designed to study the effect of synthesized electrocatalyst on the RCPE at different applied voltages with varying time. The influence of applied voltage and time on the performance of the process was examined and results are explained well.
2. Experimental
2.1. Materials
Graphite plates (1.5 × 2.5 cm2) were procured from Sunrise Enterprises, Mumbai. Sodium bicarbonate (NaHCO3), potassium bicarbonate (KHCO3), sodium carbonate (Na2CO3), potassium carbonate (K2CO3), lead nitrate (Pb(NO3)2), acetone (CH3COCH3) and iso-propyl alcohol ((CH3)2CHOH) were procured from Merck, India. Nafion (5 wt%) solution was purchased from DuPont, USA. All the chemicals were used without any further purification and deionized water was used in all the experiments.2.2. Synthesis of Pb2O and Co3O4 powder electrochemically
Lead oxide (Pb2O) powder was synthesized by electrodeposition method.28–30 The schematic for the synthesis of Pb2O is shown in Fig. 1. Catalyst powder was extracted from the solution of 0.1 M Pb(NO3)2 by using a current source in an electrolytic cell containing anode (lead metal plate) and cathode (graphite plate). The lead deposition takes place on the cathode surface on applying a constant current of 0.2 A for 3 min. Catalyst was removed from the graphite surface using acetone. Further, catalyst solution was heated at the 100 °C for 1 h to obtain Pb2O powder. Co3O4 powder was synthesized using electrodeposition method. The process involved extraction of Co from its nitrate solution (0.1 M Co (NO3)2·6H2O). A 2-elelctrode cell was used to conduct electrodeposition experiments using copper and graphite plates as anode and cathode, respectively. Deposition of Co on the surface of cathode takes place at constant applied current of 0.2 A for 3 min between two electrodes. Acetone was used to remove deposited catalyst from the surface. Co3O4 powder was obtained by heating at 100 °C for 1 h.2.3. Characterization
Synthesized electrocatalyst was characterized using Fourier Transform Infrared Spectrophotometer (FTIR, make: Shimadzu; model: IR Affinity-1) recorded in the range of 500–4000 cm−1 by crushing the sample with KBr (IR grade). X-ray diffractometer (XRD; make: Bruker; model: D8 advance) analysis was done between 10° to 80° 2θ. Particle size analysis of synthesized electrocatalysts was done using Delsa nano (make: Beckman coulter; model: Delsa nano C) particle size analyzer.2.4. Preparation of electrodes
Surface of graphite plates was coated with the synthesized electrocatalysts to fabricate anode (Co3O4) and cathode (Pb2O) electrode. Catalyst inks was prepared by taking 200 μL of (Nafion + iso-propyl alcohol) solution at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio in which 7.5 mg of electrocatalyst was added and sonicated for 30 min. Ink was coated on graphite plates to get an active area of 2 mg cm−2 at 80 °C and dried for 2 h at 100 °C each to obtain fully prepared electrode.
5 ratio in which 7.5 mg of electrocatalyst was added and sonicated for 30 min. Ink was coated on graphite plates to get an active area of 2 mg cm−2 at 80 °C and dried for 2 h at 100 °C each to obtain fully prepared electrode.
2.5. Reduction of CO2 to products electrochemically (RCPE)
Experiments in the RCPE process was performed by a two electrode system in the electrochemical glass cell. Schematic diagram of the entire process is shown in Fig. 2. CO2 gas was bubbled in 0.5 M electrolyte solutions (KHCO3, NaHCO3, K2CO3 and Na2CO3) separately, for 50 min to get CO2 saturated solution. CO2 was electrolytically reduced at cathode under voltage of 1.5 to 3.5 V and reaction samples were collected at fixed applied voltage for every time interval of 5, 10, 15, 20 and 25 min for analysis.2.6. Products analysis from RCPE
Formic acid was observed as the only product in the present reaction for all the electrolytes considered herein. Products obtained were analyzed using ultra-fast liquid chromatography (UFLC, Shimadzu LC-20AD with UV-detector of deuterium lamp SPD-20A). Reacted solution of 20 μL was taken as source sample which was injected through C-18 column (10 × 4 mm), mobile phase: 5 mM tetrabutyl ammonium hydrogen sulfate, flow rate: 1 mL min−1 at 205 nm wavelength. Faradaic efficiency was calculated using charge utilized for a particular product to the total charge utilized for the overall reaction. The formation of various products including HCOOH was confirmed by using ultrafast liquid chromatography (UFLC).3. Results and discussion
3.1. Characterization and mechanism of Pb2O electrocatalyst
FTIR spectrum of synthesized electrocatalyst was represented in Fig. 3a. The broadband around 3000–3600 cm−1 and 1642 cm−1 corresponds to O–H stretching vibrations and O–H bending vibrations, respectively, which may be due to moisture content on electrocatalyst surface. Band at 1242 cm−1 confirms the presence of Pb2O.31 XRD patterns of synthesized electrocatalyst are shown in Fig. 3b. Peak positions at 31.2°, 36.3°, 52.2°, 62.1° and 65.2° are matched closely to Pb2O structure.32 Particle size distribution of synthesized catalyst is shown in Fig. 3c. The particle size of Pb2O was found in the range of 68–295 nm. The distribution median size (Dv50) of Pb2O electrocatalyst particle was found to be 107 nm. A mechanism for the formation of Pb2O electrocatalyst is shown in Fig. 4. Lead ion from the electrolyte solution was deposited on the cathode surface by accepting electrons generated at anode (oxidation). However, driving force for the deposition was due to the newly formed Pb++ at anode into solution. Deposition is directly proportional to the formation of a new lead nitrate molecule in solution. Further, upon heating the deposited Pb in presence of oxygen gives Pb2O electrocatalyst. | ||
| Fig. 3 Characterization of synthesized Pb2O electrocatalyst, (a) FTIR, (b) XRD, and (c) particle size analysis. | ||
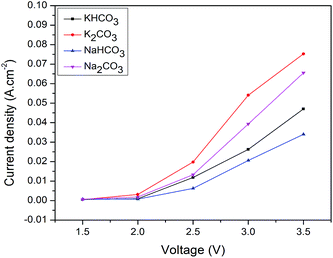
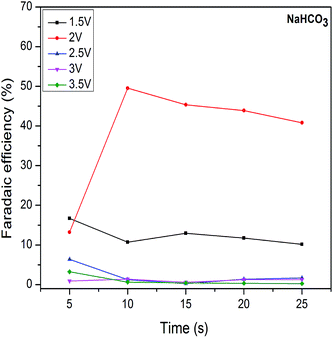
3.2. RCPE in different electrolytes solutions
 | ||
| Fig. 9 Effect of Faradaic efficiency vs. time for RCPE in Na2CO3 solution at different applied voltages. | ||
A comparison of the maximum Faradaic efficiency of HCOOH with time from RCPE using various electrolytes is shown in Table 1. From the table it may be concluded that HCOOH formation is most favorable reaction for synthesized electrocatalyst. Optimized experimental conditions to get high HCOOH Faradaic efficiency in RCPE have shown with respect to the applied voltage. However, maximum Faradaic efficiencies were obtained for bicarbonates than carbonate electrolyte solutions.
| Voltage (V) | Maximum Faradaic efficiency (time) | |||||||
|---|---|---|---|---|---|---|---|---|
| KHCO3 | K2CO3 | NaHCO3 | Na2CO3 | |||||
| (%) | (min) | (%) | (min) | (%) | (min) | (%) | (min) | |
| 1.5 | 37.27 | 15 | 21.11 | 25 | 16.68 | 5 | 21.13 | 10 |
| 2 | 58.71 | 10 | 12.56 | 5 | 49.52 | 10 | 18.79 | 5 |
| 2.5 | 1.53 | 10 | 3.71 | 5 | 6.37 | 5 | 4.54 | 5 |
| 3 | 1.47 | 5 | 1.03 | 5 | 1.37 | 5 | 4.8 | 5 |
| 3.5 | 0.98 | 5 | 0.79 | 5 | 3.23 | 5 | 1.22 | 10 |
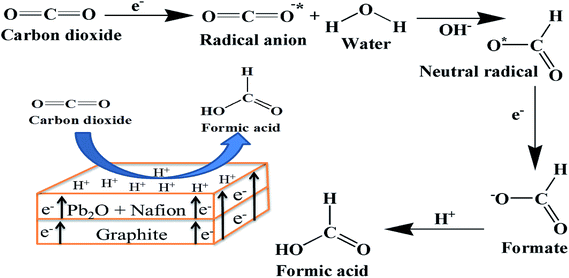
A mechanism for the formation of HCOOH by CO2 reduction on Pb2O electrocatalyst is shown in Fig. 10. It starts with accepting electron from Pb2O and adsorbs on it to form CO2 radical anion. Further, formation of formate starts with water molecule which may protonate the formed radical anion to form neutral radical. Thus neutral radical accepts new electron with some internal arrangements to form formate. The formate takes H+ to form HCOOH.32
Finally, this study describes the electrochemical reduction of CO2 to get formic acid. The investigation is on experimental phase and environmental assessment studies of the impacts that the electrochemical conversion of CO2 to formic acid produces is required. Although, RCPE is efficiently used to produce formic acid is an attractive process from the environmental point of view, but it is ambiguous that this makes up for the higher energy consumption.
4. Conclusion
Pb2O and Co3O4 were synthesized to achieve enhanced RCPE performance. The effect of electrocatalyst towards CO2 reduction is studied in presence of carbonates and bicarbonates of sodium and potassium electrolyte solutions. Results showed that only HCOOH was formed at all applied voltages. Maximum Faradaic efficiencies towards HCOOH were obtained for bicarbonate than carbonate solutions at low applied voltages. For KHCO3, at 1.5 V and 2 V high Faradaic efficiencies of 37% and 60% were observed which is the most optimum condition for RCPE. Similarly, Faradaic efficiencies of 17% and 50% were observed at reaction time of 5 and 10 min at applied voltages of 1.5 V and 2 V in NaHCO3. This preliminary investigation will be helpful to improve RCPE towards high Faradic efficiency and electrode stability for future applications.References
- M. Alvarez-Guerra, S. Quintanilla and A. Irabien, Chem. Eng. J., 2012, 207–208, 278–284 CrossRef CAS.
- I. Ganesh, Renewable Sustainable Energy Rev., 2014, 31, 221–257 CrossRef CAS.
- M. R. Goncalves, A. Gomes, J. Condeco, R. Fernandes, T. Pardal, C. A. C. Sequeira and J. B. Branco, Energy Convers. Manage., 2010, 51, 30–32 CrossRef CAS.
- Y. Hori, H. Konishi, T. Futamura, a. Murata, O. Koga, H. Sakurai and K. Oguma, Electrochim. Acta, 2005, 50, 5354–5369 CrossRef CAS.
- M. Gattrell, N. Gupta and A. Co, J. Electroanal. Chem., 2006, 594, 1–19 CrossRef CAS.
- S. Kaneco, H. Katsumata, T. Suzuki and K. Ohta, Appl. Catal., B, 2006, 64, 139–145 CrossRef CAS.
- Y. P. Peng, Y. T. Yeh, P. Y. Wang and C. P. Huang, Sep. Purif. Technol., 2013, 117, 3–11 CrossRef CAS.
- D. Kim, S. Lee, J. D. Ocon, B. Jeong, J. K. Lee and J. Lee, Phys. Chem. Chem. Phys., 2015, 17, 824–830 RSC.
- S. R. Narayanan, B. Haines, J. Soler and T. I. Valdez, J. Electrochem. Soc., 2011, 158(2), A167–A173 CrossRef CAS.
- Y. Oh and X. Hu, Chem. Soc. Rev., 2013, 42, 2253–2261 RSC.
- M. Gattrell, N. Gupta and a. Co, Energy Convers. Manage., 2007, 48, 1255–1265 CrossRef CAS.
- A. A. Peterson, F. Abild-Pedersen, F. Studt, J. Rossmeisl and J. K. Norskov, Energy Environ. Sci., 2010, 3, 1311–1315 CAS.
- J. H. Jeon, P. M. Mareeswaran, C. H. Choi and S. I. Woo, RSC Adv., 2014, 4, 3016–3019 RSC.
- N. Sreekanth and K. L. Phani, Chem. Commun., 2014, 50, 11143–11146 RSC.
- R. J. Lim, M. Xie, M. A. Sk, J. M. Lee, A. Fisher, X. Wang and K. H. Lim, Catal. Today, 2014, 233, 169–180 CrossRef CAS.
- J. Qiao, Y. Liu, F. Hong and J. Zhang, Chem. Soc. Rev., 2014, 43, 631–675 RSC.
- S. Kaneco, H. Katsumata, T. Suzuki and K. Ohta, Energy Fuels, 2006, 20, 409–414 CrossRef CAS.
- H. Yano, F. Shirai, M. Nakayama and K. Ogura, J. Electroanal. Chem., 2002, 519, 93–100 CrossRef CAS.
- Y. Hori, I. Takahashi, O. Koga and N. Hoshi, J. Mol. Catal. A: Chem., 2003, 199, 39–47 CrossRef CAS.
- M. Jitaru, D. A. Lowy, M. Toma, B. C. Toma and L. Oniciu, J. Appl. Electrochem., 1997, 27, 875–889 CrossRef CAS.
- Y. Hori, K. Kikutchi and S. Suzuki, Chem. Lett., 1985, 1695–1698 CrossRef CAS.
- F. Koleli, T. Atilan, N. Palamut, A. M. Gizir, R. Aydin and C. H. Hamann, J. Appl. Electrochem., 2003, 447–450 CrossRef.
- M. Alvarez-Guerra, S. Quintanilla and A. Irabien, Chem. Eng. J., 2012, 207–208, 278–284 CrossRef CAS.
- B. Innocent, D. Liaigre, D. Pasquier, F. Ropital, J. M. Leger and K. B. Kokoh, J. Appl. Electrochem., 2008, 39, 227–232 CrossRef.
- H. Zhao, J. Yang, L. Wang, C. Tian, B. Jiang and H. Fu, Chem. Commun., 2011, 47, 2014–2016 RSC.
- K. Subramanian, K. Asokan, D. Jeevarathinam and M. Chandrasekaran, J. Appl. Electrochem., 2007, 37, 255–260 CrossRef CAS.
- S. Yusuf and F. Jiao, Catalysis, 2012, 2, 2753–2760 CAS.
- P. B. Mathur, Proc. Indian Natl. Sci. Acad., Part A, 1982, 48, 284–289 CAS.
- Francis J. Schmidt, Philadelphia, Pa., US Pat., 3,213,004, Oct, 1965.
- T. M. Garakani, P. Norouzi, M. Hamzehloo and M. R. Ganjali, Int. J. Electrochem. Sci., 2012, 7, 857–874 CAS.
- S. N. M. Yusoff, A. Kamari, W. P. Putra, C. F. Ishak, A. Mohamed, N. Hashim and I. M. Isa, J. Environ. Prot., 2014, 5, 289–300 CrossRef CAS.
- S. J. Wang, H. Zhang, L. Shao, S. Liu and P. He, Chemosphere, 2014, 117, 353–359 CrossRef CAS PubMed.
- A. Gennaro, A. A. Isse, M. Severin, E. Vianello, I. Bhugan and J. Seveant, J. Chem. Soc., Faraday Trans., 1996, 92, 3963–3968 RSC.
- M. N. Mahmood, D. Masheder and C. J. Harty, J. Appl. Electrochem., 1987, 17, 1159–1170 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |