Highly stable oil-in-water emulsions with a gemini amphiphilic pseudopeptide†
Abstract
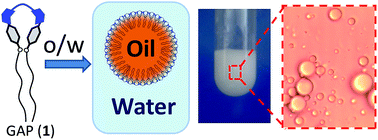
A gemini amphiphilic pseudopeptide promotes the spontaneous formation of an oil-in-water emulsion with a high thermal, mechanical and acid-medium stability. The micro-droplets thus formed are disassembled by strong bases or after the action of an enzyme, showing a potential for stimulus-responsive material formulations.

 Please wait while we load your content...
Please wait while we load your content...