High temperature CO2 sensing properties and mechanism of nanocrystalline LaCrO3 with rhombohedral structure: experiments and ab initio calculations
Abstract
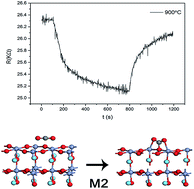
LaCrO3 nanocrystalline powders with perovskite structure were prepared by sol–gel method with annealing at 700 °C, 900 °C for 4 h, respectively. At room temperature, LaCrO3 nanocrystalline powders crystallizes as the orthorhombic structure. At high temperatures above the transition temperature from orthorhombic to rhombohedral, when exposed to CO2 the optimum operating temperature for sensing is 360 °C and the resistance of the rhombohedral LaCrO3 sensor decreases, which is different from other p-type semiconductors. First principles calculations (GGA + U) were performed to investigate the possible CO2 sensing mechanism for rhombohedral LaCrO3 sensor. The calculation results show that when exposed to CO2, the C atom of CO2 molecule chemically binds with the surface O atom of rhombohedral LaCrO3 (012) surface, meanwhile the two O atoms of CO2 molecule chemically bind with the adjacent surface Cr atoms on LaCrO3 (012) surface. For this case, the CO2 molecule captures electrons from the LaCrO3 (012) surface, which is consistent with the experimental observation.

 Please wait while we load your content...
Please wait while we load your content...