A versatile bio-inspired material platform for catalytic applications: micron-sized “buckyball-shaped” TiO2 structures
Abstract
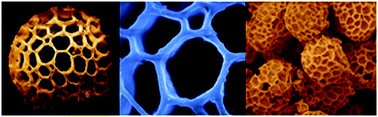
A simple sol–gel synthesis method is presented for the production of micron-sized buckyball-like TiO2 architectures using naturally occurring Lycopodium clavatum (LC) spores as biotemplates. We demonstrate that by simply altering the calcination temperature and titanium(IV) isopropoxide : ethanol volume ratio, the crystal structure and surface composition of the buckyball-like TiO2 overlayer can be readily fine-tuned. After the removal of the biological scaffold, the unique surface morphology and pore structure of the LC biotemplate can be successfully transferred to the inorganic TiO2 overlayer. We also utilize photocatalytic degradation of Rhodamine B dye samples to demonstrate the photocatalytic functionality of these micron-sized buckyball-like TiO2 architectures. Moreover, we show that the photocatalytic activity of TiO2 overlayers can be modified in a controlled manner by varying the relative surface coverages of anatase and rutile domains. These results open a potential gateway for the synthesis of a variety of bio-inspired materials with unique surface properties and shapes comprised of reducible metal oxides, metal sulfides, mixed-metal oxides, and/or perovskites.

 Please wait while we load your content...
Please wait while we load your content...