DOI:
10.1039/C5RA01528F
(Paper)
RSC Adv., 2015,
5, 45492-45501
Synthesis and evaluation of the biological activity of N′-[2-oxo-1,2 dihydro-3H-indol-3-ylidene] benzohydrazides as potential anticancer agents†
Received
29th January 2015
, Accepted 12th May 2015
First published on 13th May 2015
Abstract
New N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide derivatives were synthesized and evaluated for their cytotoxic properties against murine leukemia, L1210, human leukemia, REH and K562, human T-cell leukemia, CEM and human cervix carcinoma, HeLa cells. Among the tested compounds, the 3,4,5-trimethoxy-N′-[5-methyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide derivative (5t) emerged as the most potent inhibitor against all the tumor cell lines evaluated. To investigate the mechanism of action, 5t was further studied by cell cycle analysis, mitochondrial membrane potential analysis, DNA fragmentation and Annexin V-FITC flow cytometric analysis, which suggested that 5t was able to induce apoptosis at submicromolar range.
1. Introduction
Cancer is the most wide-spread disease across the world today. Chemotherapy is one of the main methods used for cancer treatment. The majority of drugs currently used for the treatment of cancer are cytotoxic, targeting various pathways in the cell, for example DNA repair pathways.1–4 Most of the cytotoxic drugs which are under clinical trial are of potent activity with submicromolar range. Therefore, it will be of great importance to design and develop new compounds with lower IC50 values.
Various compounds having general formula 1 display anticancer activities.5 Hydrazide derivatives possess an interesting biological activity such as anti-platelet,6 analgesic,7,8 antimicrobial,9 anti-inflammatory,10 and anticancer 211 activities. Various acyclic thiosemicarbazones 3 are lethal to mice at doses of 25–100 mg kg−1.12 The isatin-based hydrazones were reported as antioxidants,13 commercial herbicides and acetohydroxy acid synthase inhibitors,14 and beta-secretase-1 (BACE1) inhibitors.15 The hydrazide (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–NH–CO–) moiety has an important role as part of antitumor agents 2.16–18
N–NH–CO–) moiety has an important role as part of antitumor agents 2.16–18
Previously, we have reported isatin-based thiosemicarbazide derivatives as cytotoxic agents 4.19 The objectives of the present investigation were to develop analogs of 3 and 4 as potent cytotoxic agents. The derivatives 3 and 4 display cytotoxicity to human CEM T-lymphocytes and their IC50 values were high, viz, 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 2300 nM, respectively. The derivative 4 was more potent, because of insertion of polar atoms like carbonyl in place of the methylene functional group. From this basic design, we inserted an oxygen atom instead of sulfur as in isatin-hydrazones 5 and evaluated their cytotoxicity in human and murine tumor cell lines (Fig. 1). Moreover the possible underlying mechanism of action (MOA) has also been investigated.
000 and 2300 nM, respectively. The derivative 4 was more potent, because of insertion of polar atoms like carbonyl in place of the methylene functional group. From this basic design, we inserted an oxygen atom instead of sulfur as in isatin-hydrazones 5 and evaluated their cytotoxicity in human and murine tumor cell lines (Fig. 1). Moreover the possible underlying mechanism of action (MOA) has also been investigated.
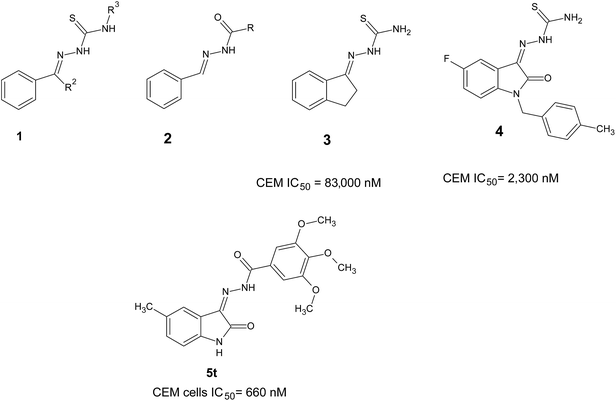 |
| | Fig. 1 Structures of various thiosemicarbazones and hydrazides. | |
2. Experimental
2.1. Chemicals and reagents
The melting points are uncorrected. The IR spectra were recorded in KBr on a Jasco 430+; the 1H NMR spectra were recorded in CDCl3/DMSO on a Bruker (400 MHz), and J values were reported in hertz (Hz). Mass spectra were recorded in triple quadrapole LCMS-6410 from Agilent technologies. 5-Cl-isatin,20 5-F-isatin,21 5-CH3-isatin,22 5-NO2-isatin,23 4-Cl-phenyl hydrazide,24 4-Br-phenyl hydrazide,25 4-NO2-phenyl hydrazide,26 4-OCH3-phenyl hydrazide27 and 3,4,5-tri-OCH3-phenyl hydrazide28 were prepared according to the literature. 4-Bromo-N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide (5b) 4-nitro-N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide (5c) and N′-[5-nitro-2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide (5x) were prepared according to the literature.29 While compounds N′-[5-chloro-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-4-methoxybenzo-hydrazide (5i) and N′-[5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide (5n) were prepared according to literature.30
2.2. General procedure for the synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide (5a–y)
A mixture of the 1H-indole-2,3-dione (I, 0.005 mol), aryl hydrazone (II, 0.007 mol) and ethanol (100 ml) was heated under reflux until the reaction was completed (∼4 h). Approximately half of the ethanol was removed in vacuo and the solution was left overnight at room temperature. The solid which precipitated was collected, washed with cold ethanol and recrystallized from ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform (9
chloroform (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the following compounds.
1) to give the following compounds.
2.2.1. 4-Chloro-N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide (5a). Obtained according to the general procedure for the synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide-yield 75%, MP > 300 °C; molecular formula C15H10ClN3O2, molecular weight 299.71, IR: 3218, 3163, 3052, 3009, 2918, 2845, 1692, 1670, 1586, 1593, 1539, 1480, 1414, 1379, 1309, 1271, 1164. 1H NMR: 13.85 (1H, s, NH), 11.41 (1H, s, NH), 7.92–7.89 (3H, dm, J = 8), 7.71 (2H, d, J = 8), 7.44–7.41 (1H, m, ar), 7.27–7.22 (1H, m, ar), 6.98–6.94 (1H, m, ar).
2.2.2. 4-Methoxy-N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide (5d). Obtained according to the general procedure for the synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide-yield 74%, MP 268–270 °C; molecular formula C16H13N3O3, molecular weight 295.29, IR: 3289, 3165, 3065, 2936, 2833, 1692, 1586, 1523, 1492, 1414, 1330, 1263, 1127. NMR: 13.87 (1H, s, NH), 11.33 (1H, s, NH), 7.88 (2H, d, J = 8), 7.60 (1H, d, J = 8), 7.38 (1H, t, J = 16), 7.15–7.09 (3H, dm, J = 8), 6.96 (1H, d, J = 8).
2.2.3. 3,4,5-Trimethoxy-N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide (5e). Obtained according to the general procedure for the synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide-yield 77%, MP 276–278 °C; molecular formula C18H17N3O5, molecular weight 355.34, IR: 3268, 3199, 3065, 2942, 2836, 1677, 1589, 1539, 1499, 1465, 1333. NMR: 13.91 (1H, s, NH), 11.31 (1H, s, NH), 7.62 (1H, d, J = 8), 7.40 (1H, t, J = 16), 7.19 (2H, s, ar), 7.12 (1H, t, J = 16), 6.98 (1H, d, J = 8), 3.87 (6H, s, 2-OCH3), 3.76 (3H, s, OCH3). MS (ESI) m/z: 354.00 (355.34).
2.2.4. 4-Chloro-N′-[5-chloro-2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide (5f). Obtained according to the general procedure for the synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide-yield 74%, MP > 300 °C; molecular formula C15H9Cl2N3O2, molecular weight 334.15, IR: 3231, 3187, 3047, 2917, 2849, 1686, 1593, 1461, 1262, 1147. NMR: 13.81 (1H, s, NH), 11.49 (1H, s, NH), 7.92 (2H, d, J = 8), 7.71 (2H, d, J = 8), 7.59 (1H, s, ar), 7.45–7.43 (1H, m, ar.), 6.99 (1H, d, J = 8). MS (ESI) m/z: 332.00 (334.15).
2.2.5. 4-Bromo-N′-[5-chloro-2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide (5g). Obtained according to the general procedure for the synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide-yield 75%, MP 288–290 °C; molecular formula C15H9BrClN3O2, molecular weight 378.60, IR: 3228, 3047, 2918, 2851, 1686, 1592, 1531, 1462, 1308, 1261, 1146. NMR: 13.81 (1H, s, NH.), 11.48 (1H, s, NH), 7.86–7.81 (4H, m, ar), 7.59 (1H, s, ar), 7.56 (1H, s, ar), 7.45–7.43 (1H, m, ar), 6.99 (2H, d, J = 8). MS (ESI) m/z: 380.00 (378.60).
2.2.6. N′-[5-Chloro-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-4-nitrobenzohydrazide (5h). Obtained according to the general procedure for the synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide-yield 72%, MP > 300 °C; molecular formula C15H9ClN4O4, molecular weight 344.70, IR: 3247, 3175, 2917, 2849, 1708, 1681, 1601, 1525, 1462, 1352, 1316. NMR: 13.93 (1H, s, NH), 11.57 (1H, s, NH), 8.42 (2H, d, J = 8), 8.14 (3H, dm, J = 8), 7.46–7.43 (1H, m, ar), 6.99 (1H, d, J = 8). MS (ESI) m/z: 343.00 (344.70).
2.2.7. N′-[5-Chloro-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-3,4,5-trimethoxy-benzohydrazide (5j). Obtained according to the general procedure for the synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide-yield 67%, MP > 300 °C; molecular formula C18H16ClN3O5, molecular weight 389.78, IR: 3265, 3108, 3047, 2942, 2835, 1710, 1661, 1580, 1527, 1492, 1333. NMR: 13.87 (1H, s, NH), 11.42 (1H, s, NH), 7.61 (1H, s, ar), 7.45–7.42 (1H, m, ar), 7.19 (2H, s, ar), 7.00 (1H, d, J = 8), 3.87 (6H, s, 2-OCH3), 3.76 (3H, s, OCH3). MS (ESI) m/z: 390.10 (389.78).
2.2.8. 4-Chloro-N′-[5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide (5k). Obtained according to the general procedure for the synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide-yield 75%, MP > 300 °C; molecular formula C15H9ClFN3O2, molecular weight 317.70, IR: 3219, 3055, 2922, 2850, 1715, 1671, 1594, 1540, 1480, 1309, 1271, 1164. NMR: 13.87 (1H, s, NH.), 11.39 (1H, s, NH), 7.92 (2H, d, J = 8), 7.71 (2H, d, J = 8), 7.43 (1H, s, ar), 7.27–7.22 (1H, m, ar), 6.98–6.95 (1H, m, ar). MS (ESI) m/z: 316.10 (317.70).
2.2.9. 4-Bromo-N′-[5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide (5l). Obtained according to the general procedure for the synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide-yield 75%, MP > 300 °C; molecular formula C15H9BrN3O2, molecular weight 362.15, IR: 3216, 3062, 2925, 2850, 1720, 1669, 1590, 1539, 1479, 1390, 1270, 1162. NMR: 13.87 (1H, s, NH.), 11.39 (1H, s, NH), 7.83 (4H, s, ar), 7.44–7.42 (1H, m, ar), 7.27–7.22 (1H, m, ar), 6.98–6.95 (1H, m, ar). MS (ESI) m/z: 362.00 (362.15).
2.2.10. N′-[5-Fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-4-nitrobenzohydrazide (5m). Obtained according to the general procedure for the synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide-yield 76%, MP > 300 °C; molecular formula C15H9FN4O4, molecular weight 328.25, IR: 3222, 3059, 2918, 2849, 1674, 1599, 1519, 1479, 1275. NMR: 13.91 (1H, s, NH), 11.41 (1H, s, NH), 8.44 (2H, d, J = 8), 8.14 (2H, d, J = 8), 7.45 (1H, s, ar), 7.28–7.23 (1H, m, ar), 6.98–6.95 (1H, m, ar). MS (ESI) m/z: 327.10 (328.25).
2.2.11. N′-[5-Fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-3,4,5-trimethoxybenzohydrazide (5o). Obtained according to the general procedure for the synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide-yield 71%, MP 228–230 °C; molecular formula C18H16FN3O5, molecular weight 373.33, IR: 3229, 3065, 2949, 1674, 1587, 1532, 1482, 1332, 1129. NMR: 13.95 (1H, s, NH), 11.35 (1H, s, NH), 7.48–7.45 (1H, m, ar), 7.29–7.23 (1H, m, ar), 7.22 (2H, s, ar), 7.01–6.98 (1H, s, ar), 3.89 (6H, s, 2-OCH3), 3.78 (3H, s, OCH3). MS (ESI) m/z: 374.33 (373.33).
2.2.12. 4-Chloro-N′-[5-methyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide (5p). Obtained according to the general procedure for the synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide-yield 75%, MP > 300 °C; molecular formula C16H12ClN3O2, molecular weight 313.73, IR: 3230, 3040, 2919, 2851, 1709, 1671, 1599, 1482, 1378, 1273, 1140. NMR: 13.90 (1H, s, NH), 11.26 (1H, s, NH), 7.91 (2H, d, J = 8), 7.70 (2H, d, J = 8), 7.43 (1H, s, ar), 7.22 (1H, d, J = 8) 6.86 (1H, d, J = 8), 2.31 (3H, s, CH3). MS (ESI) m/z: 314.10 (313.73).
2.2.13. 4-Bromo-N′-[5-methyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide (5q). Obtained according to the general procedure for the synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide-yield 77%, MP > 300 °C; molecular formula C16H12BrN3O2, molecular weight 358.18, IR: 3232, 3040, 2919, 2851, 1708, 1671, 1594, 1525, 1481, 1378, 1273. NMR: 13.88 (1H, s, NH), 11.25 (1H, s, NH), 7.82 (4H, m, ar), 7.42 (1H, s, ar), 7.21 (1H, d, J = 8), 6.85 (1H, d, J = 8), 2.30 (3H, s, CH3). MS (ESI) m/z: 358.00 (358.18).
2.2.14. N′-[5-methyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-4-nitrobenzohydrazide (5r). Obtained according to the general procedure for the synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide-yield 74%, MP > 300 °C; molecular formula C16H12N4O4, molecular weight 324.29, IR: 3256, 3113, 2918, 2851, 1702, 1677, 1601, 1523, 1339, 1140. NMR: 14.01 (1H, s, NH), 11.31 (1H, s, NH), 8.45 (2H, d, J = 8), 8.13 (2H, d, J = 8), 7.43 (1H, s, br), 7.23 (1H, d, J = 8), 6.87 (1H, d, J = 8), 2.31 (3H, s, CH3). MS (ESI) m/z: 323.10 (324.29).
2.2.15. N′-[5-methyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide (5s). Obtained according to the general procedure for the synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide-yield 71%, MP > 300 °C; molecular formula C16H13N3O2, molecular weight 279.29, IR: 3232, 3036, 2915, 2848, 1703, 1673, 1594, 1522, 1481, 1382, 1139. NMR: 13.81 (1H, s, NH), 11.25 (1H, s, NH), 7.84–7.79 (5H, m, ar), 7.42 (1H, s, ar), 7.21 (1H, d, J = 8) 6.86 (1H, d, J = 8), 2.30 (3H, s, CH3).
2.2.16. 3,4,5-Trimethoxy-N′-[5-methyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-benzohydrazide (5t). Obtained according to the general procedure for the synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide-yield 74%, MP 150 °C; molecular formula C19H19N3O5, molecular weight 369.37, IR: 3502, 3137, 3097, 2919, 1675, 1564, 1357, 1154. NMR: 13.92 (1H, s, NH.), 11.20 (1H, s, NH), 7.45 (1H, s, ar), 7.21–7.18 (3H, m, ar), 6.87 (1H, d, J = 8), 3.86 (6H, s, 2-OCH3), 3.76 (3H, s, OCH3), 2.31 (3H, s, CH3). MS (ESI) m/z: 368.10 (369.37).
2.2.17. 4-Chloro-N′-[5-nitro-2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide (5u). Obtained according to the general procedure for the synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide-yield 76%, MP > 300 °C; molecular formula C15H9ClN4O4, molecular weight 344.70, IR: 3176, 3108, 2918, 2849, 1706, 1676, 1601, 1528, 1467. NMR: 13.69 (1H, s, NH.), 12.03 (1H, s, NH), 8.34–8.29 (2H, m, ar), 7.94 (2H, d, J = 8), 7.74 (2H, d, J = 8), 7.18 (1H, d, J = 8). MS (ESI) m/z: 343.00 (344.70).
2.2.18. 4-Bromo-N′-[5-nitro-2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide (5v). Obtained according to the general procedure for the synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide-yield 71%, MP > 300 °C; molecular formula C15H9BrN4O4, molecular weight 389.16, IR: 3176, 3108, 2845, 1706, 1674, 1593, 1528, 1466, 1340, 1217. NMR: 13.69 (1H, s, NH.), 12.03 (1H, s, NH), 8.34–8.29 (2H, m, ar), 7.89–7.84 (4H, m, ar), 7.19 (1H, d, J = 8). MS (ESI) m/z: 388.90 (389.16).
2.2.19. 4-Nitro-N′-[5-nitro-2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide (5w). Obtained according to the general procedure for the synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide-yield 75%, MP > 300 °C; molecular formula C15H9N5O6, molecular weight 355.26, IR: 3192, 3108, 2925, 1707, 1681, 1603, 1523, 1343, 1146. NMR: 13.68 (1H, s, NH), 12.02 (1H, s, NH), 8.46 (2H, d, J = 8), 8.34–8.31 (2H, m, ar), 8.29 (1H, s, ar), 8.16 (2H, d, J = 8), 7.19 (1H, d, J = 8). MS (ESI) m/z: 354.10 (355.26).
2.2.20. 3,4,5-Trimethoxy-N′-[5-nitro-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-benzohydrazide (5y). Obtained according to the general procedure for the synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide-yield 70%, MP 298–300 °C; molecular formula C18H16N4O7, molecular weight 400.34, IR: 3144, 3089, 2949, 2918, 1702, 1662, 1585, 1336, 1132. NMR: 13.70 (1H, s, NH.), 11.92 (1H, s, NH), 8.32–8.30 (2H, m, ar), 7.22–7.17 (3H, m, ar), 3.88 (6H, s, 2-OCH3), 3.77 (3H, s, OCH3). MS (ESI) m/z: 399.10 (400.34).
2.3. Biological evaluations
2.3.1. Cell cultures. Human cell lines K562 (chronic myelogenous leukemia), REH (B-cell leukemia) were cultured in RPMI1640 (Sera Lab, UK) containing 10% FBS (Gibco BRL, USA) along with 100 U of penicillin G per ml as well as 100 μg of streptomycin per ml (Sigma-Aldrich, USA) at 37 °C in a humidified atmosphere containing 5% CO2.
2.3.2. Cytostatic assays. The methodology for undertaking the antiproliferative assays has been published previously.31 In brief, varying concentrations of the compounds (5-fold dilutions) were incubated at 37 °C for 72 h (HeLa and CEM T-lymphocytes) or 48 h (L1210 cells) in 200 μl 96-well microtiter plates, and the viable tumor cell number was counted at the end of the incubation period using a Coulter Counter (Coulter Electronics, Harpenden Hertz, U.K.).
2.3.3. Trypan blue dye exclusion assay. The viability of the leukemic cells (REH, K562) were determined by trypan blue dye exclusion assay after treatment with 5t.32 Cells were cultured (0.5 × 105 cells per ml) for 24 h and 5t was added at different concentration (0–10 μM). DMSO treated cells were used as vehicle control. Cells were collected at 48 and 72 h after treatment with 5t and viable cells were counted following trypan blue staining. Each experiment was repeated three independent times and standard error mean was calculated and plotted with error bars.
2.3.4. MTT assay. The proliferation rate of the cells after treatment with 5t was checked by using MTT assay.33 REH, K562 cells (0.5 × 105 cells per ml) were treated with 5t and MTT assay was carried out after 48 and 72 h incubation. Cells treated with equal amount of DMSO were used as vehicle control. Experiment was repeated three independent times each with duplicate reactions and bar diagram was plotted with error bars.
2.3.5. Cell cycle analysis. Effect of 5t on cell cycle was checked as described previously.34 Briefly, REH cells were cultured (0.5 × 105 cells per ml), and after 24 h incubation, 5t was added (0, 0.1 and 0.5 μM), cells were harvested after 24 and 48 h of treatment, washed, fixed with 70% ethanol and RNase-A treatment was given overnight, finally analyzed in BD FACSVerse™ flow cytometer using propidium iodide stain. A minimum of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells were acquired per sample and histograms were plotted and analyzed using flowing software (version 2.5). Experiment was repeated two independent times and each cell cycle phase was indicated in bar diagram with error bar.
000 cells were acquired per sample and histograms were plotted and analyzed using flowing software (version 2.5). Experiment was repeated two independent times and each cell cycle phase was indicated in bar diagram with error bar.
2.3.6. Measurement of the mitochondrial membrane potential. Mitochondrial transmembrane potential was measured using JC-1 (5, 5′, 6, 6′-tetrachloro-1, 1, 3, 3′-tetraethylbenzimidazolcarbocyanamide iodide; Calbiochem, USA) dye after treatment with 5t. Briefly, after 48 h of treatment with 5t at 0, 0.1 and 0.5 μM concentration, cells were harvested, and incubated in the media containing 0.5 μM JC-1 for 20 min at 37 °C, cells were washed in phosphate buffered saline and analyzed in (FACS Calibur, BD Biosciences, USA) flow cytometer. 2,4-DNP was used as a positive control.35 Green (low mitochondrial membrane potential) versus red (high mitochondrial membrane potential) ratio of JC-1 florescence was calculated using flowing software (version2.5) and represented in a bar diagram with error bar using two independent experiments.
2.3.7. Annexin V-FITC flow cytometric analysis. Movement of phosphatidylserine to outer membrane upon apoptotic induction was detected by Annexin V-FITC staining (Santacruz, USA).36 REH cells were treated with 5t (0, 0.1 and 0.5 μM) for 48 h and DMSO treated cells were used as vehicle control, cells were harvested, washed with cold 1× phosphate buffered saline and re-suspended in binding buffer (HEPES-10 mM pH 7.4, 144 mM NaCl and 25 mM CaCl2), cells were incubated with Annexin V-FITC (200 μg per ml) and propidium iodide (10 μg per ml) for 20 min at room temperature and analyzed in BD FACSVerse™ flow cytometer. A minimum of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells were acquired per sample and represented in dot plot. Experiment was repeated two independent times and bar diagram was plotted for early apoptosis, late apoptosis and necrosis with error bars.
000 cells were acquired per sample and represented in dot plot. Experiment was repeated two independent times and bar diagram was plotted for early apoptosis, late apoptosis and necrosis with error bars.
2.3.8. DNA fragmentation assay. The fragmentation of DNA after treatment with 5t was checked using agarose gel electrophoresis.37,38 Briefly, REH cells were treated with 5t in different concentration (0, 0.1, 0.5 and 1 μM) for 48 h, DMSO treated cells were used as a vehicle control. Cells were harvested and total genomic DNA was isolated using standard protocol, and dissolved in 1X TE, loaded on 2% agarose gel and run at 50 v for 3 h. DNA marker was used to check the length of fragmented DNA ladder.
2.3.9. Detection of intracellular ROS production by flow cytometer. The total intracellular ROS production after treatment with 5t was determined using cell permeable fluorescent probe 2, 7-dichlorodihydro fluorescein diacetate (H2DCFDA) in REH cells.39 Briefly, REH cells were treated with 0.5 μM of 5t for different time points (5, 15, 30 and 60 min), cells were harvested, washed with 1× phosphate buffered saline and incubated with 0.5 μM H2DCFDA for 37 °C for 15 minutes and the green fluorescence intensity was analyzed by flow cytometry (FACS Calibur, BD Biosciences, USA). Cells which are treated with H2O2 were used as positive control. The shift in the peak after treatment with 5t was compared with control and percentage of ROS production was determined.
2.3.10. Statistical analysis. The values were expressed as mean ± SEM for control and experimental samples and statistical analysis was performed using one-way ANOVA followed by the Dunnett test. Significance was mentioned after comparing treated samples with control. For this analysis, GraphPad software prism 5.1 was used. The values were considered as statistically significant, if the p-value was equal to or less than 0.05.
3. Results and discussion
3.1. Chemistry
The synthesis of the N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide derivatives (5a–y) from 1H-indole-2,3-dione I and the benzohydrazide II has been outlined in Scheme 1. The chemical structures of the newly synthesized 5a–y were established on the basis of analytical and spectroscopic data. In the IR spectra, the absorption for carbonyl groups and NH bands were observed in the range of 1720–1661 cm−1 and 3289–3165 cm−1, respectively. In the 1H-NMR spectra, the chemical shifts showed for ring NH between δ = 13.95–13.66 ppm and for amide NH between 12.02–11.19 ppm in the form of singlet. The aromatic protons signals appeared in the range of δ = 8.46–6.85 ppm. The signals for CH3 and OCH3 were observed in the range of δ = 2.31–2.30 and 3.89–3.76 ppm, respectively. Furthermore, all compounds were confirmed by ESI-MS and were in agreement with their molecular weight.
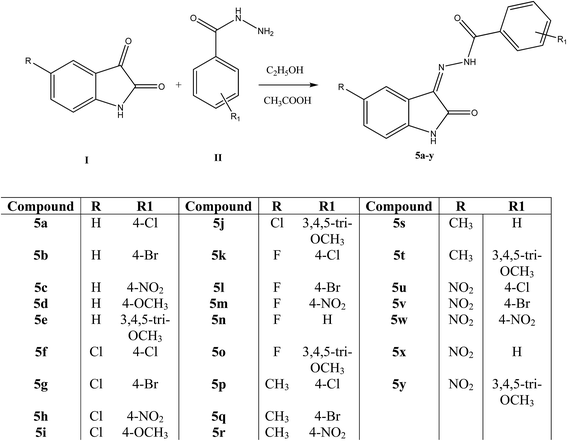 |
| | Scheme 1 Synthesis of N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide derivatives (5a–y). | |
3.2. Biological evaluation
3.2.1. Cytotoxicity in human and murine tumor cell cultures. The evaluation of the compounds in Scheme 1 towards human cervix carcinoma (HeLa), T-lymphocyte (CEM) and murine leukemia (L1210) cells was undertaken in order to determine whether the compounds were cytotoxic to human cancer cells.The cytotoxicity of the compounds in series 5a–y was determined and melphalan was used as a standard compound (Table 1). There was no improvement in cytotoxic activity of compounds 5a–c, 5f–p and 5u–y. Their IC50 values were >250 μM, except for derivative 5g. When hydrogen was substituted with electron-withdrawing groups like halogen (Cl/F) and nitro (NO2) at the 5th position of the isatin moiety resulting in the formation of compounds 5f–o and 5u–y, the cytotoxic activity was poor towards all cell lines. These studies suggest that electron-withdrawing substitution is not favorable for either the isatin or the phenyl groups.
Table 1 Inhibitory effects of derivatives of N′-[2-oxo-1,2-dihydro-3H-indol-3-ylidene]benzohydrazide derivatives (5a–y) on the proliferation of murine leukemia (L1210), human T-lymphocyte (CEM) and cervix carcinoma (HeLa) cells
| Code |
IC50a (μM) |
| L1210 |
CEM |
HeLa |
| 50% inhibitory concentration. NT-not tested. |
| 5a |
>250 |
>250 |
>250 |
| 5b |
>250 |
>250 |
>250 |
| 5c |
>250 |
>250 |
>250 |
| 5d |
31 ± 21 |
10 ± 5 |
22 ± 4 |
| 5e |
35 ± 29 |
6.7 ± 0.9 |
59 ± 11 |
| 5f |
>250 |
>250 |
>250 |
| 5g |
70 ± 35 |
31 ± 7 |
19 ± 2 |
| 5h |
>250 |
>250 |
>250 |
| 5i |
>250 |
>250 |
>250 |
| 5j |
>250 |
>250 |
>250 |
| 5k |
>250 |
>250 |
>250 |
| 5l |
>250 |
>250 |
>250 |
| 5m |
>250 |
>250 |
>250 |
| 5n |
>250 |
>250 |
>250 |
| 5o |
>250 |
>250 |
>250 |
| 5p |
190 ± 11 |
114 ± 1 |
130 ± 13 |
| 5q |
33 ± 2 |
16 ± 8 |
23 ± 4 |
| 5r |
>250 |
>250 |
>250 |
| 5s |
18 ± 4 |
6.3 ± 0.9 |
22 ± 0 |
| 5t |
0.90 ± 0.06 |
0.66 ± 0.23 |
1.0 ± 0.2 |
| 5u |
147 ± 10 |
138 ± 22 |
100 ± 40 |
| 5v |
>250 |
>250 |
>250 |
| 5w |
>250 |
>250 |
>250 |
| 5x |
138 ± 11 |
131 ± 8 |
125 ± 16 |
| 5y |
130 ± 2 |
>250 |
>250 |
| Melphalan |
2.13 ± 0.02 |
1.4 ± 0.4 |
NT |
However, when the methyl and methoxy groups were substituted on the isatin and phenyl groups, respectively, resulting in the formation of the derivatives 5p–t, there was much improvement in cytotoxicity towards all three cell lines (IC50 33–0.66 μM) except for compound 5r (IC50 > 250 μM). The structure–activity relationship study suggests that electron-donating groups such as methyl and methoxy (5d, 5e, 5q, 5s and 5t) are preferred over electron-withdrawing substituents such as halogens (5f–o) and the nitro (5u–y) group. Among the tested compounds, 5t was clearly the more potent in the L1210, CEM and HeLa tumor cell assays [sub micromolar range].
The data therefore provided ample evidence for pursuing derivative 5t for further study. As the lead compound 5t showed significant cytotoxicity against leukemic cells, we further carried out the studies to understand the mechanism of action in different leukemia cells, namely REH and K562.
3.2.2. Induction of cytotoxicity in REH and K562 cells. To evaluate the cytotoxicity exerted by 5t, we used the leukemic cell lines, REH and K562. B-cell leukemia cells (REH) showed a very good response towards treatment of 5t and we found that the IC50 value was around 0.5 μM (Fig. 2A and B). At the same time, chronic myelogenous leukemia cells (K562) showed a moderate response to 5t and the IC50 value was found to be ∼2 μM (Fig. 2C and D).
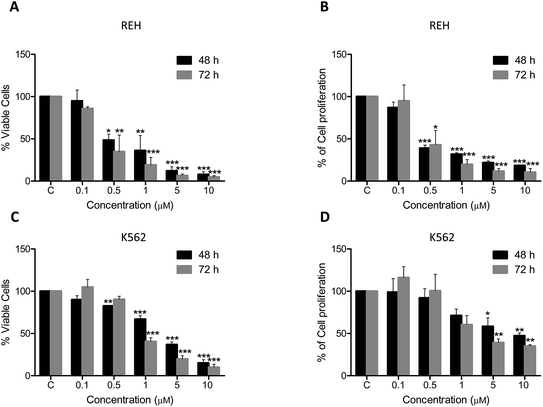 |
| | Fig. 2 Cytotoxic effect of 5t on REH and K562 cells. REH and K562 cells were seeded (0.5 × 105 cells per ml) and 5t (0–10 μM) was added after 24 h. Trypan blue assay (to detect the number of viable cells) and MTT assay (to check proliferation rate) were performed at 48 and 72 h of treatment. A. Trypan blue assay for 5t treated REH cells. B. MTT assay for 5t treated REH cells. C. Trypan blue assay for 5t treated K562 cells. D. MTT assay for 5t treated K562 cells. The cells treated with equal concentration of DMSO was used as vehicle control and in all the cases (indicated by ‘C’). Each experiment was performed three independent times and error bars are indicated. | |
3.2.3. Effect of 5t on cell cycle progression. To check whether 5t affects cell cycle progression, REH cells were treated with 5t at different concentrations (0, 0.1 and 0.5 μM) for 24 and 48 h. The cells were harvested and analyzed by flow cytometry after staining with propidium iodide. The results showed no cell cycle arrest at tested concentration and time point, however, significant accumulation of cells in the SubG1 phase was observed in a concentration dependent manner (Fig. 3A and B).
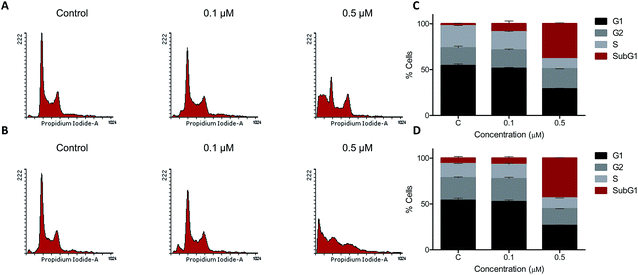 |
| | Fig. 3 Cell cycle analysis of 5t treated REH cells. REH cells were seeded (0.5 × 105 cells per ml) and were treated with 5t (0, 0.1 and 0.5 μM) after 24 h. Cells were harvested after 24 and 48 h of treatment, fixed in 70% ethanol, washed and stained with propidium iodide and analyzed in flow cytometer. A and B. Histogram representing effect on REH cell cycle by 5t at 24 and 48 h respectively. C and D. The bar graph representing different phases of cell cycle in REH cells after treatment with 5t at 24 and 48 h respectively. Error bars were plotted from two independent experiments. | |
3.2.4. Effect of 5t on the mitochondrial membrane potential. Mitochondria play a major role in induction of apoptosis.40 To check the mitochondrial membrane potential which is very important for maintaining the integrity of the cell, we treated REH cells with 5t (100 and 500 nM for 48 h), stained with the JC1 dye and analyzed the cells by FACS. Interestingly, we observed disruption of the mitochondrial membrane potential in a concentration dependent manner, which suggests that 5t induced apoptosis by disruption of the mitochondrial membrane potential (Fig. 4A and B).
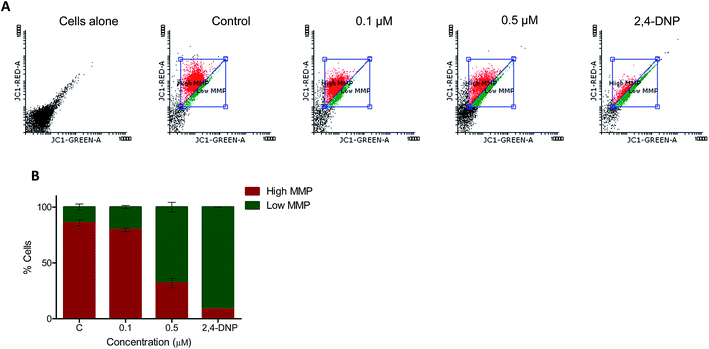 |
| | Fig. 4 Measurement of mitochondrial membrane potential. Total mitochondrial membrane potential after treatment with 5t was measured by using JC-1 dye. REH cells were treated with 5t (0, 0.1 and 0.5 μM) for 48 h. Cells were harvested, stained with JC-1 dye at 37 °C for 20 min and analyzed in flow cytometer. 2,4-DNP treated cells were used as a positive control, negative control is DMSO treated cells without JC-1 staining and JC-1 stained DMSO treated cells served as control. A. Dot plot showing control as well as 5t treated REH cells. B. Bar diagram showing red (high MMP) and green (low MMP) fluorescence ratio of JC-1 with error bars. | |
3.2.5. Annexin-V-FITC flow cytometry. To check the nature of cell death, annexin-V-FITC staining was done for REH cells after treatment with 0.1 and 0.5 μM of 5t at the 48 h time-point. Results showed an increased number of annexin-V-FITC-positive cells after treatment with 5t, which clearly suggested the apoptotic mode of cell death (Fig. 5A and B).
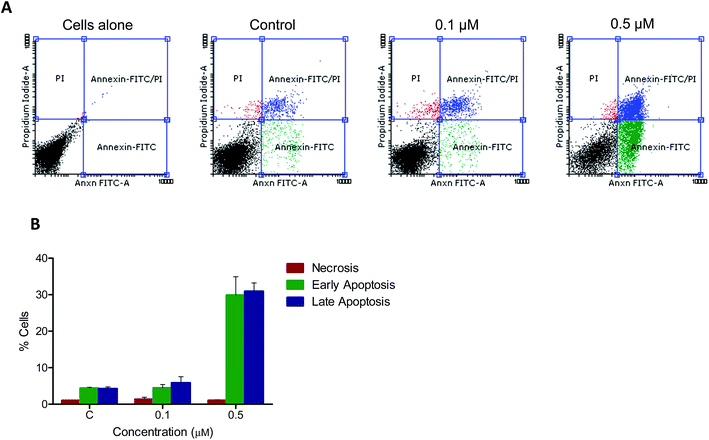 |
| | Fig. 5 Annexin V-FITC flow cytometry. Apoptotic signals from 5t treated REH cells was detected using Annexin V-FITC staining. After 48 h of treatment with 5t (0, 0.1 and 0.5 μM) cells were harvested, and stained with both Annexin V-FITC and propidium iodide for 20 minutes and analyzed in flow cytometer. DMSO treated cells were used as vehicle control. A. Dot plot representing control and 5t treated cells. B. Bar diagram representing early apoptotic, late apoptotic and necrotic population of control as well as treated cells. Experiment was repeated minimum two times and error bar was represented. | |
3.2.6. DNA fragmentation assay. Fragmentation of DNA by activated endonucleases inside the cell at the onset of drug treatment is one of the markers for apoptosis.41 Therefore, we investigated the possibility of DNA fragmentation in REH cells after 48 h of treatment with 5t (0, 0.1, 0.5 and 1 μM). Interestingly, the treatment of 5t resulted in a fragmented DNA ladder formation in a concentration-dependent manner from 0.5 μM onwards, which suggested possible apoptotic induction upon 5t treatment (Fig. 6A). Both DNA fragmentation and Annexin-V-FITC staining confirmed the induction of apoptosis in REH cells after the treatment with 5t.
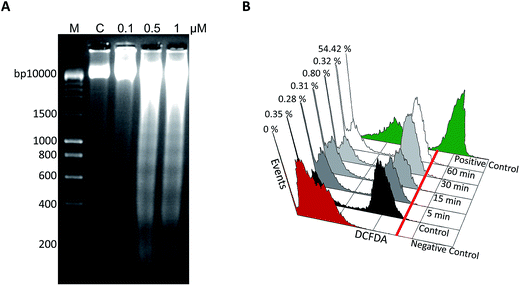 |
| | Fig. 6 Detection of DNA fragmentation and ROS generation in 5t treated REH cells. A. REH cells were treated with 5t (0, 0.1, 0.5 and 1 μM for 48 h) and cells were harvested, genomic DNA was isolated and subjected to agarose gel electrophoresis. DMSO treated cells were used as vehicle control. Samples were run on a 2% agarose gel (50 v for 3 h) along with the DNA marker. ‘C’ represents the DMSO treated samples, ‘M’ represents the DNA marker lane. B. REH cells were seeded (0.5 × 105 cells per ml) and treated with 0.5 μM of 5t for different time points (5, 15, 30 and 60 min). Cells were harvested and stained with 0.5 μM H2DCFDA for 15 min at 37 °C. ROS production was analyzed using flow cytometer. Positive control indicates H2O2 treated cells. Cells without H2DCFDA served as a negative control and DMSO treated cells with H2DCFDA was served as control. % cells which are positive for green fluorescent of ROS were measured. | |
3.2.7. Effect of 5t on intracellular ROS production. Generation of reactive oxygen species by inducing cell death or apoptosis is one of the mechanisms of action of many drugs. To examine the generation of ROS, REH cells were treated with 5t (0.5 μM) at different time points such as 5, 15, 30 and 60 minutes. Cells treated with H2O2 served as a positive control. The results showed no ROS generation by 5t at the selected time points (Fig. 6B), which suggested 5t induced cell death is independent of ROS production.
4. Conclusion
In summary, a series of 25 isatin-hydrazone derivatives with various structural features were synthesized and their cytotoxicity was evaluated against human cervix carcinoma, T-lymphocyte and murine leukemia cell lines. The structure activity relationship studies indicated that a methyl group on the isatin and a 3,4,5-trimethoxy substituents on the benzene are required for pronounced cytotoxicity (5s, 5q and 5t). Therefore, derivative 5t was selected for further studies to understand the mechanism of action in two different leukemic cell lines, REH and K562. The cell cycle study, analysis of mitochondrial membrane potential and Annexin V-FITC using flow cytometry suggested that 5t was able to induce apoptosis without arresting the cell cycle. In summary, 5t, compound with potent anticancer activity could be studied further and developed as an anticancer agent.
Acknowledgements
We gratefully acknowledge the support for this research effort from All India Council for Technical Education (AICTE), New Delhi (ref. no. 8023/BOR/RID/RPS-169/2008-09) for SSK. The cytotoxic activities were determined by financial support of the KU Leuven (GOA 10/014). We would also like to thank the NMR facility, FACS facility, Indian Institute of Science, Bangalore for characterization of all compounds. We thank Lizette van Berckelaer for the cytostatic determinations.
References
- M. Srivastava and S. C. Raghavan, Chem. Biol., 2015, 22, 17 CrossRef CAS PubMed.
- M. Srivastava, M. Nambiar, S. Sharma, S. S. Karki, G. Goldsmith, M. Hegde, S. Kumar, M. Pandey, R. K. Singh, P. Ray, R. Natarajan, M. Kelkar, A. De, B. Choudhary and S. C. Raghavan, Cell, 2012, 151, 1474 CrossRef CAS PubMed.
- F. John, J. George, S. V. Vartak, M. Srivastava, P. A. Hassan, V. K. Aswal, S. S. Karki and S. C. Raghavan, Macromol. Biosci., 2015, 15, 521 CrossRef CAS PubMed.
- X. Chen, S. Zhong, X. Zhu, B. Dziegielewska, T. Ellenberger, G. M. Wilson, A. D. Jr MacKerell and A. E. Tomkinson, Cancer Res., 2015, 68, 3169 CrossRef PubMed.
- V. E. Anatoly, S. E. Rashel, J. L. Margeris, A. Z. Aina, Z. D. Anda and M. K. Iya, Chem. Abstr., 1985, 102, 78567j Search PubMed , FR 2540868, August 17, 1984.
- A. C. Cunha, J. M. Figueiredo, J. L. M. Tributino, A. L. P. Miranda, H. C. Castro, R. B. Zingali, C. A. M. Fraga, M. C. B. V. de Souza, V. F. Ferreira and E. J. Barreiro, Bioorg. Med. Chem., 2003, 11, 2051 CrossRef CAS.
- L. F. C. C. Leite, M. N. Ramos, J. B. P. da Silva, A. L. P. Miranda, C. A. M. Fraga and E. J. Barreiro, Farmaco, 1999, 54, 747 CrossRef CAS.
- P. C. Lima, L. M. Lima, K. C. M. da Silva, P. H. O. Léda, A. L. P. de Miranda, C. A. M. Fraga and E. J. Barreiro, Eur. J. Med. Chem., 2000, 35, 187 CrossRef CAS.
- C. Loncle, J. M. Brunel, N. Vidal, M. Dherbomez and Y. Letourneux, Eur. J. Med. Chem., 2004, 39, 1067 CrossRef CAS PubMed.
- A. R. Todeschini, A. L. P. de Miranda, K. C. M. da Silva, S. C. Parrini and E. J. Barreiro, Eur. J. Med. Chem., 1998, 33, 189 CrossRef CAS.
- N. Terzioglu and A. Gürsoy, Eur. J. Med. Chem., 2003, 38, 781 CrossRef CAS.
- J. R. Dimmock, D. C. Smith, J. M. Brenner, S. S. Jonnalagadda, M. S. Sardessai, J. D. Wood and G. E. Bigam, Eur. J. Med. Chem., 1986, 21, 187 CAS.
- M. Katyal and Y. Dutt, Talanta, 1975, 22, 151 CrossRef CAS.
- J. Wang, H. Tan, Y. Li, Y. Ma, Z. Li, L. W. Guddat and J. Agric, Food Chem., 2011, 59, 9892 CrossRef CAS PubMed.
- N. Y. Mok, J. Chadwick, K. A. B. Kellett, N. M. Hooper, A. P. Johnson and C. W. G. Fishwick, Bioorg. Med. Chem. Lett., 2009, 19, 6770 CrossRef CAS PubMed.
- P. Vicini, M. Incerti, I. A. Doytchinova, P. La Colla, B. Busonera and R. Loddo, Eur. J. Med. Chem., 2006, 41, 624 CrossRef CAS PubMed.
- H. Abadi, A. A. H. Eissa and G. S. Hassan, Chem. Pharm. Bull., 2003, 51, 838 CrossRef.
- D. Kumar, N. M. Kumar, S. Ghosh and K. Shah, Bioorg. Med. Chem. Lett., 2012, 22, 212 CrossRef CAS PubMed.
- S. S. Karki, V. S. Bahaduria, V. Rana, S. Kumar, G. S. Prasanna, U. Das, J. Balzarini, E. De Clercq and J. R. Dimmock, J. Enzyme Inhib. Med. Chem., 2009, 24, 537 CrossRef CAS PubMed.
- K. Jin, X. Zhang, C. Ma, Y. Xu, Y. Yuan and W. Xu, Bioorg. Med. Chem., 2013, 21, 2663 CrossRef CAS PubMed.
- A. Natarajan, Y.-H. Fan, H. Chen, Y. Guo, J. Iyasere, F. Harbinski, W. J. Christ, H. Aktas and J. A. Halperin, J. Med. Chem., 2004, 47, 1882 CrossRef CAS PubMed.
- L. Hua-Quan, W. De-Cai, W. Fei, T. Wei and O. Ping-Kai, Chin. Chem. Lett., 2013, 24, 929 CrossRef PubMed.
- R. W. Daisley and V. K. Shah, J. Pharm. Sci., 1984, 73, 407 CrossRef CAS PubMed.
- S. D. Jorge, F. Palace-Berl, A. Masunari, C. A. Cechinel, M. Ishii, K. F. M. Pasqualoto and L. C. Tavares, Bioorg. Med. Chem., 2011, 19, 5031 CrossRef CAS PubMed.
- T. Medwick and H. M. Abdou, J. Pharm. Sci., 1979, 68, 301 CrossRef CAS PubMed.
- N. P. Peet and S. Sunder, J. Heterocycl. Chem., 1984, 21, 1807 CrossRef CAS PubMed.
- N. P. Grimster, S. Connelly, A. Baranczak, J. Dong, L. B. Krasnova, K. B. Sharpless, E. T. Powers, I. A. Wilson and J. W. Kelly, J. Am. Chem. Soc., 2013, 135, 5656 CrossRef CAS PubMed.
- K. Sung and L. An-Rong, J. Heterocycl. Chem., 1992, 29, 1101 CrossRef CAS PubMed.
- K. Debnath, S. Pathak and A. Pramanik, Tetrahedron Lett., 2013, 54, 4110 CrossRef CAS PubMed.
- Z. Liang, D. Zhang, J. Ai, L. Chen, H. Wang, X. Kong, M. Zheng, H. Liu, C. Luo, M. Geng, H. Jiang and K. Chen, Bioorg. Med. Chem. Lett., 2011, 21, 3749 CrossRef CAS PubMed.
- P. B. Baraldi, N. M. DelC, M. A. Tabrizo, E. D. Clercq, J. Balzarini, J. Bermejo, F. Estevez and R. Romagnoli, J. Med. Chem., 2004, 47, 2877 CrossRef CAS PubMed.
- B. T. Moorthy, S. Ravi, M. Srivastava, K. K. Chiruvella, H. Hemlal, O. Joy and S. C. Raghavan, Bioorg. Med. Chem. Lett., 2010, 20, 6297 CrossRef CAS PubMed.
- S. Kumar, V. Gopalakrishnan, M. Hegde, V. Rana, S. S. Dhepe, S. A. Ramareddy, A. Leoni, A. Locatelli, R. Morigi, M. Rambaldi, M. Srivastava, S. C. Raghavan and S. S. Karki, Bioorg. Med. Chem. Lett., 2014, 24, 4682 CrossRef CAS PubMed.
- S. Sharma, K. Panjamurthy, B. Choudhary, M. Srivastava, M. Shahabuddin, R. Giri, G. M. Advirao and S. C. Raghavan, Mol. Carcinog., 2013, 52, 413 CrossRef CAS PubMed.
- S. S. Karki, K. Panjamurthy, S. Kumar, M. Nambiar, S. A. Ramareddy, K. K. Chiruvella and S. C. Raghavan, Eur. J. Med. Chem., 2011, 46, 2109 CrossRef CAS PubMed.
- K. K. Chiruvella, K. Panjamurthy, B. Choudhary, O. Joy and S. C. Raghavan, Int. J. Biomed. Sci., 2010, 6(3), 182–194 CAS.
- U. Sahu, H. Sidhar, P. S. Ghate, G. M. Advirao, S. C. Raghavan and R. K. Giri, PLoS One, 2013, 8, e66430 CAS.
- S. Kumar, M. Hegde, V. Gopalakrishnan, V. K. Renuka, S. A. Ramareddy, E. D. Clercq, D. Schols, G. N. Anilkumar, S. C. Raghavan and S. S. Karki, Eur. J. Med. Chem., 2014, 84, 687 CrossRef CAS PubMed.
- M. Hegde, S. S. Karki, E. Thomas, S. Kumar, K. Panjamurthy, S. R. Ranganatha, K. S. Rangappa, B. Choudhary and S. C. Raghavan, PLoS One, 2012, 7, e43632 CAS.
- C. Wang and R. J. Youle, Annu. Rev. Genet., 2009, 43, 95 CrossRef CAS PubMed.
- Y. Gavrieli, Y. Sherman and S. A. Ben-Sasson, J. Cell Biol., 1992, 119, 493 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra01528f |
| ‡ Equal first authors. |
|
| This journal is © The Royal Society of Chemistry 2015 |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–NH–CO–) moiety has an important role as part of antitumor agents 2.16–18
N–NH–CO–) moiety has an important role as part of antitumor agents 2.16–18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 2300 nM, respectively. The derivative 4 was more potent, because of insertion of polar atoms like carbonyl in place of the methylene functional group. From this basic design, we inserted an oxygen atom instead of sulfur as in isatin-hydrazones 5 and evaluated their cytotoxicity in human and murine tumor cell lines (Fig. 1). Moreover the possible underlying mechanism of action (MOA) has also been investigated.
000 and 2300 nM, respectively. The derivative 4 was more potent, because of insertion of polar atoms like carbonyl in place of the methylene functional group. From this basic design, we inserted an oxygen atom instead of sulfur as in isatin-hydrazones 5 and evaluated their cytotoxicity in human and murine tumor cell lines (Fig. 1). Moreover the possible underlying mechanism of action (MOA) has also been investigated.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform (9
chloroform (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the following compounds.
1) to give the following compounds.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells were acquired per sample and histograms were plotted and analyzed using flowing software (version 2.5). Experiment was repeated two independent times and each cell cycle phase was indicated in bar diagram with error bar.
000 cells were acquired per sample and histograms were plotted and analyzed using flowing software (version 2.5). Experiment was repeated two independent times and each cell cycle phase was indicated in bar diagram with error bar.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells were acquired per sample and represented in dot plot. Experiment was repeated two independent times and bar diagram was plotted for early apoptosis, late apoptosis and necrosis with error bars.
000 cells were acquired per sample and represented in dot plot. Experiment was repeated two independent times and bar diagram was plotted for early apoptosis, late apoptosis and necrosis with error bars.






