Synthesis, stabilization, growth behavior, and catalytic activity of highly concentrated silver nanoparticles using a multifunctional polymer in an aqueous-phase†
Aasim Shahzad,
Woo-Sik Kim* and
Taekyung Yu*
Department of Chemical Engineering, College of Engineering, Kyung Hee University, Youngin, 446-701, Korea. E-mail: wskim@khu.ac.kr; tkyu@khu.ac.kr
First published on 19th March 2015
Abstract
This article proposes an initiative protocol for the aqueous-phase synthesis of highly concentrated Ag nanoparticles with long-term stability, in which the multifunctional polymer in the reaction was found to be of great importance to the formation and stabilization of Ag nanoparticles. Highly concentrated Ag nanoparticles (above 20 g L−1) synthesized by the reaction of AgNO3 with branched polyethyleneimine exhibited long-term stability without any size change or precipitation (more than 40 days). Transmission electron microscopy and UV-vis analysis revealed that aggregation of small Ag nanoparticles occurred at the very early stages of synthesis, and spherical Ag nanoparticles with sizes of around 8 nm were synthesized by coalescence and reshaping of aggregates. We also investigated the influence of reaction temperature and pH of the reacting solution on the Ag nanoparticle synthesis, demonstrating that these experimental factors are critical in terms of reaction completion time, size, size distribution, morphology, and stability of Ag nanoparticles. In addition, the resulting Ag nanoparticles exhibited effective activity in the catalytic reduction of 4-nitrophenol to 4-aminophenol by NaBH4.
Introduction
Metal nanoparticles attract interest around the globe due to their potential applications.1–7 They are useful for energy conversion, catalysis, photoelectronics, biomedicines, and device development.8–24 In particular, silver (Ag) nanoparticles have emerged as a subject of special interest due to their performance in applications such as tunable localized surface plasmon resonance, surface enhanced Raman scattering, biosensors, printed electronics, and antimicrobial technology.25–32 In order to utilize the diversity of Ag nanoparticles in fundamental studies as well as in industrial applications, mass production of high quality nanoparticles is required.33,34 Over the past decades, several research groups have reported on synthetic methods for colloidal Ag nanoparticles, but these methods still have room for improvement. For example, aqueous-phase routes for the synthesis of Ag nanoparticles by reacting Ag salt with a reducing agent in the presence of stabilizer often suffer from either very low concentration (below 10 mM) of resulting nanoparticles or aggregation of nanoparticles occurs when the synthesis is conducted at higher concentrations of Ag precursor.35–37 A polyol method, typically using ethylene glycol for both solvent and reductant, can form Ag nanoparticles with various morphologies including cubes, wires, and plates.38–40 However, ethylene glycol would not be a good chemical reagent for mass production of Ag nanoparticles due to its high price compared with water. Therefore, developing a simple and economic route for synthesizing highly concentrated Ag nanoparticles remains a significant challenge.Typically, water-based methods, which produce Ag nanoparticles via the reduction of metal salts using reductant, require multiple reagents such as reducing agents, capping agents, and/or stabilizers.41,42 Multifunctional polymers, which can act as both reducing and stabilizing agents, are key factors in the mass production of stable nanoparticles. One of the major advantages of multifunctional polymer-induced synthesis is that processes are easy to scale-up because of their simplicity. The effective steric hindrance from a long hydrocarbon chain can provide long-term stability for the nanoparticles in an aqueous-phase by preventing agglomeration. There have been some reports of the synthesis of Ag nanoparticles using a single component polymer; however, synthesized Ag nanoparticles were stable in only a water–toluene interface or they could be synthesized at Ag concentrations as low as 1 mM.43,44
In this work, we developed an alternative aqueous-phase synthesis method for highly concentrated Ag nanoparticles with long-term stability. Our proposed procedure based on branched polyethyleneimine (BPEI) as a multifunctional polymer involves the use of AgNO3, BPEI, and deionized water. There have been some reports for the synthesis of Ag nanoparticles using PEI. Signori and co-workers synthesized catalytic Ag nanoparticles supported on polyethyleneimine (PEI) derivatives. However, PEI was used as an only supporting material to immobilize Ag nanoparticles and the synthesis needed a long reaction time of 5 days.45 Tan et al. and Liong et al. also reported the syntheses of Ag nanoparticles using PEI as a stabilizer. However, these syntheses were conducted in a non-aqueous phase and sizes of resulting Ag nanoparticles were larger than 20–50 nm.46,47 In the present synthesis, the synthesized highly concentrated Ag nanoparticles (above 20 g L−1) exhibited spherical shapes with an average size of around 8 nm and long-term stability (more than 40 days) without any size change and precipitation in an aqueous-phase. The percentage yield of the synthetic reaction was also calculated using thermal gravimetric analysis (TGA) and it came out to be greater than 84%. Transmission electron microscopy (TEM) and UV-vis analysis revealed that aggregation of small Ag nanoparticles occurred at the very early stages of the synthesis, and spherical Ag nanoparticles with sizes of around 8 nm were synthesized by coalescence and reshaping of aggregates. We also investigated the influence of reaction temperature and pH of the reacting solution on Ag nanoparticle synthesis, demonstrating that these experimental factors are critical in terms of reaction completion time, size, size distribution, morphology, and stability of Ag nanoparticles. Furthermore, the Ag nanoparticles were found to exhibit effective activity in the catalytic reduction of 4-nitrophenol into 4-aminophenol by NaBH4.
Experimental section
Materials
BPEI (MW = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 750
000 and 750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 50 wt% solution in water), linear polyethyleneimine (LPEI, MW = 25
000, 50 wt% solution in water), linear polyethyleneimine (LPEI, MW = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), ethyleneimine oligomer (MW = 423), polyvinylpyrrolidone (PVP, MW = 50
000), ethyleneimine oligomer (MW = 423), polyvinylpyrrolidone (PVP, MW = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), silver nitrate (AgNO3, ≥99%), sodium hydroxide (NaOH, ≥98%), 4-nitrophenol, sodium borohydride, and nitric acid (HNO3, ∼70%) were purchased from Aldrich and used without further purification. Water was purified by ion-exchange (deionized, DI water).
000), silver nitrate (AgNO3, ≥99%), sodium hydroxide (NaOH, ≥98%), 4-nitrophenol, sodium borohydride, and nitric acid (HNO3, ∼70%) were purchased from Aldrich and used without further purification. Water was purified by ion-exchange (deionized, DI water).
Synthesis of Ag nanoparticles
In a typical synthesis, 0.169 g of BPEI (MW = 750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was dissolved in 4 ml of deionized water and heated to 50 °C in air under magnetic stirring. Meanwhile, 1 ml of 1 M aqueous AgNO3 solution was added to the reaction solution using a micropipette (final volume of the solution was 5 ml and weight ratio of BPEI/AgNO3 was 0.5). The pH of the resulting solution was 10. The resulting mixture was heated at this temperature for an appropriate reaction time while being magnetically stirred, and then cooled down to room temperature. The colloidal suspension was transferred into a 50 ml tube and centrifuged at 9000 rpm for 30 min. The final product was collected by repeating centrifugation and washing with water three times to remove the excess BPEI. The obtained Ag nanoparticles were redispersed in water.
000) was dissolved in 4 ml of deionized water and heated to 50 °C in air under magnetic stirring. Meanwhile, 1 ml of 1 M aqueous AgNO3 solution was added to the reaction solution using a micropipette (final volume of the solution was 5 ml and weight ratio of BPEI/AgNO3 was 0.5). The pH of the resulting solution was 10. The resulting mixture was heated at this temperature for an appropriate reaction time while being magnetically stirred, and then cooled down to room temperature. The colloidal suspension was transferred into a 50 ml tube and centrifuged at 9000 rpm for 30 min. The final product was collected by repeating centrifugation and washing with water three times to remove the excess BPEI. The obtained Ag nanoparticles were redispersed in water.
Catalytic study of Ag nanoparticles
The aqueous solution containing 2.65 ml of deionized water, 30 μL of an aqueous 4-nitrophenol solution (5 mM), and 100 μL of 1 M NaBH4 solution was stored into a UV-vis cuvette. Then, 20 μL of an aqueous suspension of Ag nanoparticles was added into the cuvette to start the reaction (the molar ratio of substrate/catalyst was 2). The intensity of the absorption peak at 400 nm was monitored by UV-vis spectroscopy as a function of time.Characterization
TEM and high-resolution TEM (HRTEM) images were captured using a JEM-2100F microscope operated at 200 kV. The scanning electron microscopy (SEM) images were obtained using a LEO SUPRA 55 microscope. The powder X-ray diffraction (XRD) patterns were obtained using a Rigaku D-MAX/A diffractometer at 35 kV and 35 mA. TGA was performed using a TGA Q5000 IR thermal analyzer. The UV-vis spectra were recorded using a Jasco UV-vis spectrophotometer within the range of 300–800 nm. Fourier transform infrared spectroscopy (FTIR) analysis was performed using a Jasco FTIR-6100 equipped with ATR assembly in the transmission mode. X-ray photoelectron spectroscopy (XPS) data was obtained using a Thermo Scientific K-Alpha spectrometer. Total concentration of Ag nanoparticles in the aqueous solution was measured using a direct reading Echelle inductively coupled plasma (ICP) spectrometer.Results and discussion
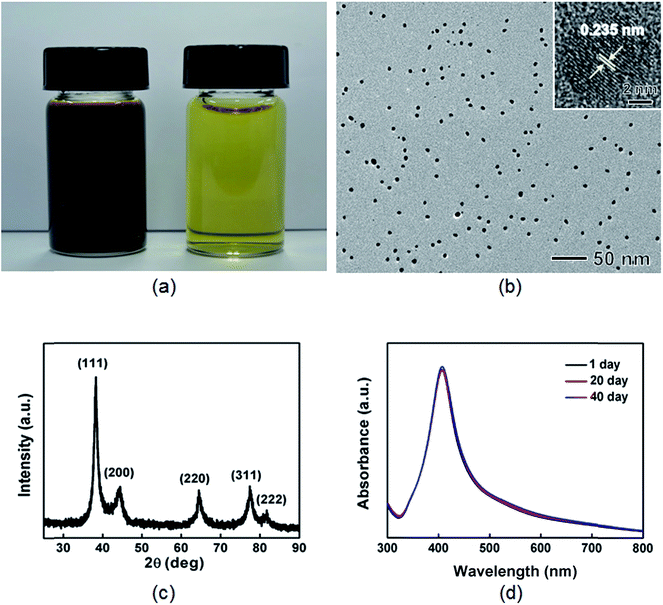
The Ag nanoparticles were synthesized by reacting silver nitrate (AgNO3) with BPEI (MW = 750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) in an aqueous-phase at 50 °C for 10 h. The BPEI could act as a both reducing and stabilizing agent, similar to the mechanism of conventional BPEI-based Au nanoparticle synthesis, as reported previously.48,49 After addition of AgNO3 to the aqueous BPEI solution, the color of the solution changed gradually from light yellow to orange yellow and finally to blackish brown, indicating the formation of colloidal Ag nanoparticles (Fig. 1(a)). A TEM image of Ag nanoparticles showed that the nanoparticles exhibited spherical shape with an average size of 8.0 nm and narrow size distribution (Fig. 1(b) and S9(b) in ESI†). The Ag nanoparticles exhibited both single-crystal and twinned structures, as can be seen in HRTEM images (inset of Fig. 1(b) and S1 in ESI†). The powder XRD patterns taken from the sample show the presence of diffraction peaks at 38.1°, 44.3°, 64.7°, 77.4°, and 81.6° which can be assigned to (111), (200), (220), (311), and (222) planes of face-centered cubic (fcc) silver (Fig. 1(c), Fm3m, a = 4.086 Å, Joint Committee on Powder Diffraction Standards (JCPDS) file no. 04-0783), respectively. We did not observe any diffraction peaks of impure crystalline phases. The XPS spectrum shows two distinct peaks at 368.2 and 374.3 eV corresponding to the binding energies of metallic Ag 3d5/2 and Ag 3d3/2 core levels, respectively, thus confirming the metallic structure of the Ag nanoparticles (Fig. S2 in ESI†). The UV-vis extinction spectrum taken from an aqueous suspension of the Ag nanoparticles shows the presence of a strong plasmon resonance peak at around 408 nm, matching well with previous reports on Ag nanoparticles with a similar particle size.50,51 In addition, the UV-vis spectrum of the aqueous suspension of Ag nanoparticles stored at room temperature for 40 days still shows a strong peak at 408 nm without any red shifting or peak broadening, indicating the absence of any size increase or the formation of aggregates (Fig. 1(d)). TEM image of Ag nanoparticles stored after 40 days also exhibits the stability of nanoparticles (Fig. S3 in ESI†). These observations demonstrate that the synthesized Ag nanoparticles have long-term stability in an aqueous-phase. The percentage yield of Ag nanoparticles was calculated by using TGA in a nitrogen atmosphere at a heating rate of 10 °C min−1. BPEI stabilized Ag nanoparticles shows around 10% weight loss at a temperature of as high as 410 °C, while BPEI shows around 100% weight loss at the same temperature (Fig. S4 in ESI†). Based on the calculation using TGA data, we could conclude that the percentage yield of Ag nanoparticles came out to be greater than 84%.
000) in an aqueous-phase at 50 °C for 10 h. The BPEI could act as a both reducing and stabilizing agent, similar to the mechanism of conventional BPEI-based Au nanoparticle synthesis, as reported previously.48,49 After addition of AgNO3 to the aqueous BPEI solution, the color of the solution changed gradually from light yellow to orange yellow and finally to blackish brown, indicating the formation of colloidal Ag nanoparticles (Fig. 1(a)). A TEM image of Ag nanoparticles showed that the nanoparticles exhibited spherical shape with an average size of 8.0 nm and narrow size distribution (Fig. 1(b) and S9(b) in ESI†). The Ag nanoparticles exhibited both single-crystal and twinned structures, as can be seen in HRTEM images (inset of Fig. 1(b) and S1 in ESI†). The powder XRD patterns taken from the sample show the presence of diffraction peaks at 38.1°, 44.3°, 64.7°, 77.4°, and 81.6° which can be assigned to (111), (200), (220), (311), and (222) planes of face-centered cubic (fcc) silver (Fig. 1(c), Fm3m, a = 4.086 Å, Joint Committee on Powder Diffraction Standards (JCPDS) file no. 04-0783), respectively. We did not observe any diffraction peaks of impure crystalline phases. The XPS spectrum shows two distinct peaks at 368.2 and 374.3 eV corresponding to the binding energies of metallic Ag 3d5/2 and Ag 3d3/2 core levels, respectively, thus confirming the metallic structure of the Ag nanoparticles (Fig. S2 in ESI†). The UV-vis extinction spectrum taken from an aqueous suspension of the Ag nanoparticles shows the presence of a strong plasmon resonance peak at around 408 nm, matching well with previous reports on Ag nanoparticles with a similar particle size.50,51 In addition, the UV-vis spectrum of the aqueous suspension of Ag nanoparticles stored at room temperature for 40 days still shows a strong peak at 408 nm without any red shifting or peak broadening, indicating the absence of any size increase or the formation of aggregates (Fig. 1(d)). TEM image of Ag nanoparticles stored after 40 days also exhibits the stability of nanoparticles (Fig. S3 in ESI†). These observations demonstrate that the synthesized Ag nanoparticles have long-term stability in an aqueous-phase. The percentage yield of Ag nanoparticles was calculated by using TGA in a nitrogen atmosphere at a heating rate of 10 °C min−1. BPEI stabilized Ag nanoparticles shows around 10% weight loss at a temperature of as high as 410 °C, while BPEI shows around 100% weight loss at the same temperature (Fig. S4 in ESI†). Based on the calculation using TGA data, we could conclude that the percentage yield of Ag nanoparticles came out to be greater than 84%.
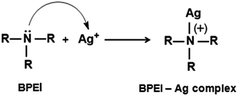
In previous syntheses of Au nanoparticles using BPEI as a reactant, it was demonstrated that a primary and secondary amine of BPEI can be used as a reducing agent to convert Au3+ to Au.48,52,53 In order to investigate the structural changes in BPEI during the reduction process, FTIR analysis was performed. A FTIR transmission spectrum of BPEI-stabilized Ag nanoparticles shows disappearance of the absorption peak at 1104 cm−1 and appearance of a distinct peak at 1645 cm−1 compared with that of unreacted BPEI, indicating the formation of a carbon–nitrogen double bond (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) originated from the oxidation of BPEI (Fig. 2).48,54 When the synthesis was conducted in the presence of ethyleneimine oligomer as a reactant instead of BPEI, large Ag aggregates were produced, confirming that an amine group can act as a reducing agent for the synthesis of Ag nanoparticles (Fig. S5 in ESI†). We also found that the weight ratio of BPEI/AgNO3 would play an important role in the formation of Ag nanoparticles. When the synthesis was conducted at a high BPEI/AgNO3 weight ratio of 55, we could not observe any specific peak in a UV-vis spectrum of Ag nanoparticles (Fig. S6 in ESI†). In previous studies for the BPEI, non-bonding electrons of the amine group could lead to the formation of a stable complex of metal cation and BPEI.55,56 A UV-vis spectrum of a sample prepared at a BPEI/AgNO3 weight ratio of 55 shows intense absorption peaks at 288 nm even after 40 h of reaction time, indicating the formation of a stable BPEI–Ag complex (Fig. S6 in ESI†).
N) originated from the oxidation of BPEI (Fig. 2).48,54 When the synthesis was conducted in the presence of ethyleneimine oligomer as a reactant instead of BPEI, large Ag aggregates were produced, confirming that an amine group can act as a reducing agent for the synthesis of Ag nanoparticles (Fig. S5 in ESI†). We also found that the weight ratio of BPEI/AgNO3 would play an important role in the formation of Ag nanoparticles. When the synthesis was conducted at a high BPEI/AgNO3 weight ratio of 55, we could not observe any specific peak in a UV-vis spectrum of Ag nanoparticles (Fig. S6 in ESI†). In previous studies for the BPEI, non-bonding electrons of the amine group could lead to the formation of a stable complex of metal cation and BPEI.55,56 A UV-vis spectrum of a sample prepared at a BPEI/AgNO3 weight ratio of 55 shows intense absorption peaks at 288 nm even after 40 h of reaction time, indicating the formation of a stable BPEI–Ag complex (Fig. S6 in ESI†).
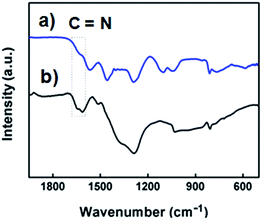 | ||
| Fig. 2 FTIR spectra of (a) BPEI and (b) the BPEI-stabilized Ag nanoparticles shown in Fig. 1. | ||
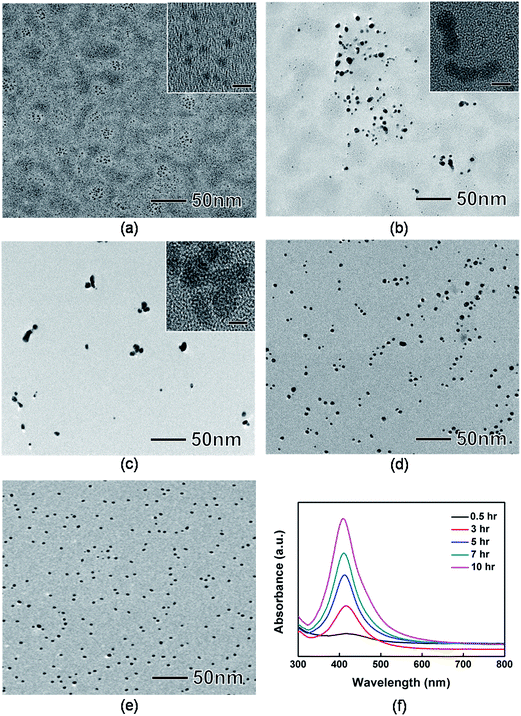
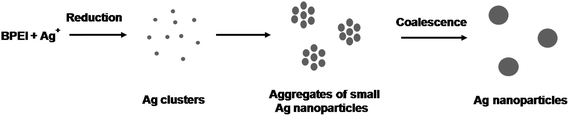
In typical syntheses of nanoparticles, monomer attachment on the surface of seed particles is a main growth factor.57 Recently, however, there have been some reports that size and morphology of nanoparticles could be modulated by particle coalescence and reshaping.58,59 We investigated the morphological evolution of the Ag nanoparticles by taking samples at various reaction stages and then analyzing them by TEM and UV-vis spectrometer. Fig. 3(a) to (e) show typical TEM and HRTEM images of the products sampled at 0.5, 3, 5, 7, and 10 h, respectively. At t = 0.5 h, 20 nm-sized aggregates composed of small Ag nanoparticles with sizes of around 2 nm were found (Fig. 3(a)). At t = 1 h, we observed peanut-shaped or elongated nanoparticles (Fig. 3(b)). The HRTEM image of the peanut-shaped nanoparticles shows a neck between adjacent nanoparticles (inset of Fig. 3(b)). This result indicates that the peanut-shaped nanoparticles was most likely synthesized via particle coalescence rather than atomic (or monomer) addition. As the reaction proceeded to t = 5 h, the Ag nanoparticles shows more branched shapes with multiple necks as shown in the HRTEM image (Fig. 3(c) and inset of Fig. 3(c)). After t = 7 h, the Ag nanoparticles started to take a spherical shape with small number of elongated ones (Fig. 3(d)). In the next 3 h, the surface of Ag nanoparticles was smoothed, showing the formation of spherical Ag nanoparticles with sizes of around 8 nm (Fig. 3(e)). To achieve a better understanding of the growth mechanism of Ag nanoparticles, UV-vis measurements were performed (Fig. 3(f)). At the early stages of synthesis, the UV-vis spectrum exhibited the presence of weak and broad absorption peaks at around 429 nm. During the synthesis, the maximum absorption peaks of Ag nanoparticles were 418 nm at t = 3 h, 412 nm at t = 5 h, 410 nm at t = 7 h, and 408 nm at t = 10 h, respectively, indicating the blue-shifting due to the size reduction of Ag nanoparticles. Based on these observation, we could conclude that the Ag nanoparticles were synthesized by coalescence and reshaping of small Ag nanoparticles (Fig. 4).
We performed detailed comparison experiments of growth behavior by varying the experimental conditions including concentration, molecular weight, morphology, and type of polymer. We could exclude the role of reagent concentration because blue-shifting was also observed in UV-vis analysis when BPEI and AgNO3 were diluted by a factor of 100 (Fig. 5(a)). We also observed blue-shifting when the synthesis was conducted in the presence of light BPEI (MW = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and linear polyethyleneimine (LPEI) instead of BPEI (MW = 750
000) and linear polyethyleneimine (LPEI) instead of BPEI (MW = 750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), respectively, confirming that molecular weight and morphology of PEI are not critical for the growth behavior of the Ag nanoparticles (Fig. 5(b) and (c)). In contrast, polyvinylpyrrolidone (PVP), which has different functional groups except for the amine group, exhibits red-shifting in UV-vis analysis when it is used as a stabilizer instead of BPEI (Fig. 5(d)). These results demonstrate that the presence of PEI is responsible for the interesting growth behavior in this synthesis. At the present time, however, the driving forces for aggregation at very early stages are not fully resolved. A complete understanding of the growth mechanism of the Ag nanoparticles using PEI may require further study using both experiments and simulations. Representative TEM images and size distribution of Ag nanoparticles with comparison experiments are shown in Fig. S7 and S8 in ESI,† respectively.
000), respectively, confirming that molecular weight and morphology of PEI are not critical for the growth behavior of the Ag nanoparticles (Fig. 5(b) and (c)). In contrast, polyvinylpyrrolidone (PVP), which has different functional groups except for the amine group, exhibits red-shifting in UV-vis analysis when it is used as a stabilizer instead of BPEI (Fig. 5(d)). These results demonstrate that the presence of PEI is responsible for the interesting growth behavior in this synthesis. At the present time, however, the driving forces for aggregation at very early stages are not fully resolved. A complete understanding of the growth mechanism of the Ag nanoparticles using PEI may require further study using both experiments and simulations. Representative TEM images and size distribution of Ag nanoparticles with comparison experiments are shown in Fig. S7 and S8 in ESI,† respectively.
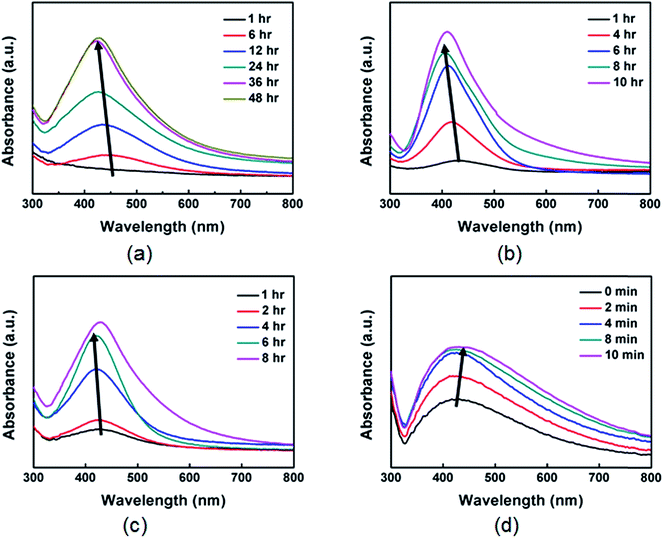 | ||
Fig. 5 UV-vis spectra of Ag nanoparticles prepared under the same conditions as those in Fig. 1, except that synthesis was conducted in the presence of (a) 100 times diluted BPEI and AgNO3, (b) BPEI having low molecular weight (MW = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), (c) LPEI (MW = 25 000), (c) LPEI (MW = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), and (d) PVP, respectively. 000), and (d) PVP, respectively. | ||
Because our synthesis is carried out in the aqueous-phase, the reaction temperature and pH of the reacting solution are also important for both formation and stabilization of the Ag nanoparticles. In a recent report on the synthesis of Au nanoparticles using BPEI, it was demonstrated that the synthetic reaction of HAuCl4 with BPEI progressed slowly due to the low activity of the BPEI amine groups at a low reaction temperature, thus leading to formation of large Au nanopoarticles.49 Average sizes of the Ag nanoparticles were 8.3 ± 2.6 nm at 30 °C, 8.0 ± 0.9 nm at 50 °C, 7.2 ± 1.2 nm at 70 °C, and 3.6 ± 0.9 nm at 90 °C, respectively, revealing that the size of the Ag nanoparticles gradually decreased with increasing reaction temperature (Fig. 6 and S9 in ESI†).49,60 At a low reaction temperature of 30 °C, nucleation was initiated at a relatively low supersaturation because of slow reduction reaction. Therefore, large portion of monomers could be used to the growth of Ag nanoparticles, thus leading the formation of large-sized Ag nanoparticles. In contrast, at a high temperature of 90 °C, because a large portion of monomers were already consumed for a nucleation stage, small Ag nanoparticles were synthesized due to the lack of monomers for growth of Ag nanoparticles. In addition, we also found that reaction time (i.e. the time to reach maximum absorbance in UV-vis spectra) was reduced by increasing the reaction temperature, from 130 h at 30 °C to 24 min at 90 °C (Fig. S10 in ESI†).
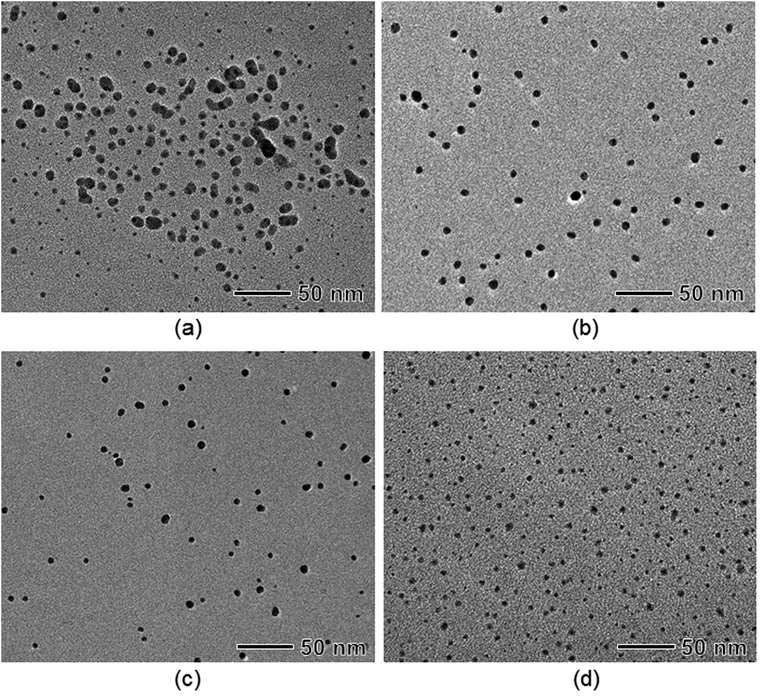 | ||
| Fig. 6 TEM images of Ag nanoparticles prepared under the same conditions as those in Fig. 1, except that synthesis was conducted at various reaction temperatures: (a) 30 °C, (b) 50 °C, (c) 70 °C, and (d) 90 °C, respectively. | ||
In the present synthesis, the reducing and stabilizing ability of BPEI strongly depends on its pH environment. When the synthesis was conducted under strong acidic conditions (pH = 3.0), precipitation occurred at the bottom of the vial. A scanning electron microscopy (SEM) image shows agglomerated particles with sizes larger than 700 nm (Fig. 7(a)). Under mild acidic conditions (pH = 5.0), the size of the synthesized Ag nanoparticles was reduced to 200 nm but they still exhibited poor stability in an aqueous solution, confirming that the amine group in PEI has a weaker ability in terms of reduction, complex formation, capping, and stabilization under acidic conditions, as reported previously (Fig. 7(b)).48 When increasing the pH value to 10 (the standard condition shown in Fig. 1), the aqueous suspension of Ag nanoparticles exhibited long-term stability for more than 40 days and the size of the Ag nanoparticles significantly decreased to 8 nm with a narrow size distribution (Fig. 7(c)). In addition, Fig. 7(d) shows that the average particle size of Ag nanoparticles increased to 12.5 nm with a relatively broad distribution under more basic conditions (pH = 13). We believe that the hydroxide ion (OH−) in the solution acted as another reducing agent to convert Ag+ to Ag.61
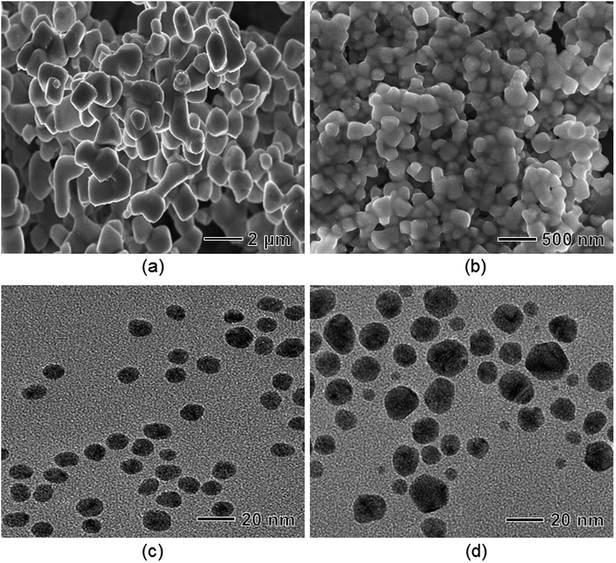 | ||
| Fig. 7 TEM images of Ag nanoparticles prepared under the same conditions as those in Fig. 1, except that synthesis was conducted at various initial pH values: (a) 3.0, (b) 5.0, (c) 10.0, and (d) 13.0, respectively. | ||
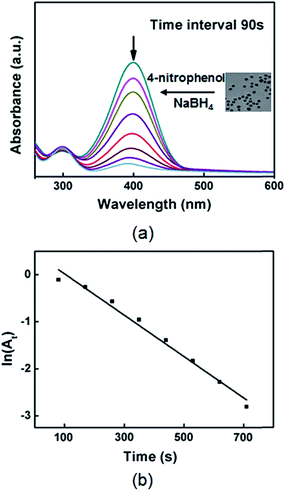
The catalytic activity of the Ag nanoparticles was studied by employing the reduction of 4-nitrophenol into 4-aminophenol by NaBH4 as a model reaction.62,63 The kinetic process of the reduction reaction could be investigated by monitoring the intensity of the absorption peak at 400 nm associated with 4-nitrophenol as a function of time. Fig. S11 in ESI† shows the UV-vis spectra of an aqueous solution containing 4-nitrophenol and NaBH4 in the absence of the Ag nanoparticles, indicating there is no reaction without the Ag nanoparticles. After the Ag nanoparticles had been added, the absorption peak at 400 nm gradually dropped in intensity as the reduction reaction proceeded, demonstrating that the Ag nanoparticles could act as a catalyst for the reduction reaction of 4-nitrophenol (Fig. 8(a)). In this study, excess amount of NaBH4 was added to the reaction so that the reaction rate could be assumed to be independent of the concentration of NaBH4, and the pseudo first order kinetics with respect to 4-nitrophenol could be applied to determine the catalytic activity of the Ag nanoparticles. Fig. 8(b) shows a good linear correlation of ln(At) against time, where At is the absorbance (at 400 nm) corresponding to the reduction time. The rate constant (k) was 4.1 × 10−3 s−1, determined from the linear plot of ln(At) versus time. To compare the catalytic activity of the Ag nanoparticles with the literature values, we used activity parameter κ (where, κ = k/m), which is the ratio of the rate constant (k) to the total mass (m) of the catalyst. The catalytic activity of the synthesized Ag nanoparticles was 454 s−1 g−1 (m = 9.02 × 10−6 g), which is quite higher than the previously reported values (Table 1).62,64−67 We think that small size (lots of catalytic sites), high surface area, and well dispersion of the Ag nanoparticles in the aqueous-phase could lead the high activity.
| Nanoparticles | Size (nm) | Catalyst (g) | Rate constant (k, s−1) | Activity parameter (K, s−1 g−1) | Ref. |
|---|---|---|---|---|---|
| TAC–Ag-1.0 | 35 | 4.0 × 10−3 | 5.19 × 10−3 | 1.29 | 62 |
| Ag–NP/C composite | 10 | 1.0 × 10−3 | 1.69 × 10−3 | 1.69 | 64 |
| CNFs/AgNPs | 25.7 | 1.0 × 10−3 | 4.60 × 10−3 | 4.60 | 65 |
| Fe3O4@SiO2–Ag | 3.65 | 1.0 × 10−3 | 7.67 × 10−3 | 7.67 | 66 |
| Fe3O4@C@Ag | 30 | 1.0 × 10−5 | 3.72 × 10−3 | 372 | 67 |
| BPEI–Ag | 8 | 9.0 × 10−6 | 4.10 × 10−3 | 454 | This work |
Conclusions
We have demonstrated an alternative aqueous-phase synthesis of highly concentrated 8 nm-sized Ag nanoparticles with long-term stability, in which the multifunctional polymer in the reaction was found to be important to the formation and stabilization of Ag nanoparticles. Highly concentrated Ag nanoparticles (above 20 g L−1) were synthesized by the reaction AgNO3 with BPEI exhibited long-term stability without any size change or precipitation over more than 40 days. The percentage yield of the synthetic reaction came out to be greater than 84%.We found that aggregation of small Ag nanoparticles occurred at the very early stages of the synthesis, and spherical Ag nanoparticles with sizes of around 8 nm were synthesized by coalescence and reshaping of aggregates. To our knowledge, this is the first report for highly concentrated BPEI stabilized Ag nanoparticles synthesized by coalescence and reshaping of aggregates. We also investigated the influence of reaction temperature and pH of the reacting solution on Ag nanoparticle synthesis, demonstrating that these experimental factors are critical in terms of reaction completion time, size, size distribution, morphology, and stability of Ag nanoparticles. In addition, the synthesized Ag nanoparticles showed good catalytic activity in the reduction of 4-nitrophenol into 4-aminophenol by NaBH4. The presented synthetic route is readily applicable to the large-scale production of Ag nanoparticles for practical applications due to its simple reaction conditions and the high concentration of nanoparticles in the resulting solution. Furthermore, we expect that this synthetic strategy can be extended to large-scale synthesis of other metal nanoparticles, including Pt, Pd, and Cu.Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2014R1A5A1009799). This work was also supported through a Mid-Career Researcher based on a National Research Foundation grant funded by the Korean Ministry of Education, Science and Technology (NRF-2010-0017993). This work was also supported by the Engineering Research Center of Excellence Program of the Korea Ministry of Science, ICT & Future Planning(MSIP)/National Research Foundation of Korea(NRF) (Grant NRF-2014-009799).References
- V. L. Colvin, M. C. Schlamp and A. P. Alivisatos, Nature, 1994, 370, 354–357 CrossRef CAS.
- M. Bruchez, M. Moronne, P. Gin, S. Weiss and A. P. Alivisatos, Science, 1998, 281, 2013–2016 CrossRef CAS.
- S. Sun, C. B. Murray, D. Weller, L. Folks and A. Moser, Science, 2000, 287, 1989–1992 CrossRef CAS.
- A. Roucoux, J. Schulz and H. Patin, Chem. Rev., 2002, 102, 3757–3778 CrossRef CAS PubMed.
- Y. Sun and Y. Xia, Science, 2002, 298, 2176–2179 CrossRef CAS PubMed.
- Y. Wang, Y. Zheng, C. Z. Huang and Y. Xia, J. Am. Chem. Soc., 2013, 135, 1941–1951 CrossRef CAS PubMed.
- A. Desireddy, B. E. Conn, J. Guo, B. Yoon, R. N. Barnett, B. M. Monahan, K. Kirschbaum, W. P. Griffith, R. L. Whetten, U. Landman and T. P. Bigioni, Nature, 2013, 501, 399–402 CrossRef CAS PubMed.
- D. L. Feldheim and C. D. Keating, Chem. Soc. Rev., 1998, 27, 1–12 RSC.
- M. Merschdorf, W. Pfeiffer, A. Thon, S. Voll and G. Gerber, Appl. Phys. A, 2000, 71, 547–552 CrossRef CAS.
- T. A. Taton, C. A. Mirkin and R. L. Letsinger, Science, 2000, 289, 1757–1760 CrossRef CAS.
- S. A. Maier, M. L. Brongersma, P. G. Kik, S. Meltzer, A. A. G. Requicha and H. A. Atwater, Adv. Mater., 2001, 13, 1501–1505 CrossRef CAS.
- P. K. Sudeep, B. I. Ipe, K. G. Thomas, M. V George, S. Barazzouk, S. Hotchandani and P. V. Kamat, Nano Lett., 2001, 2, 29–35 CrossRef.
- C. T. Campbell, S. C. Parker and D. E. Starr, Science, 2002, 298, 811–814 CrossRef CAS PubMed.
- P. V. Kamat, J. Phys. Chem. B, 2002, 106, 7729–7744 CrossRef CAS.
- S. Praharaj, S. Nath, S. K. Ghosh, S. Kundu and T. Pal, Langmuir, 2004, 20, 9889–9892 CrossRef CAS PubMed.
- E. Katz and I. Willner, Angew. Chem., Int. Ed., 2004, 43, 6042–6108 CrossRef CAS PubMed.
- D. Lee, R. E. Cohen and M. F. Rubner, Langmuir, 2005, 21, 9651–9659 CrossRef CAS PubMed.
- Y. Li, Y. Wu and B. S. Ong, J. Am. Chem. Soc., 2005, 127, 3266–3267 CrossRef CAS PubMed.
- H. Tsunoyama, H. Sakurai, Y. Negishi and T. Tsukuda, J. Am. Chem. Soc., 2005, 127, 9374–9375 CrossRef CAS PubMed.
- D. I. Enache, J. K. Edwards, P. Landon, B. Solsona-Espriu, A. F. Carley, A. A. Herzing, M. Watanabe, C. J. Kiely, D. W. Knight and G. J. Hutchings, Science, 2006, 311, 362–365 CrossRef CAS PubMed.
- P. K. Jain, K. S. Lee, I. H. El-Sayed and M. A. El-Sayed, J. Phys. Chem. B, 2006, 110, 7238–7248 CrossRef CAS PubMed.
- M. A. Noginov, G. Zhu, A. M. Belgrave, R. Bakker, V. M. Shalaev, E. E. Narimanov, S. Stout, E. Herz, T. Suteewong and U. Wiesner, Nature, 2009, 460, 1110–1112 CrossRef CAS PubMed.
- Z. Zhang, L. Zhang, M. N. Hedhili, H. Zhang and P. Wang, Nano Lett., 2012, 13, 14–20 CrossRef PubMed.
- R. R. Arvizo, S. Bhattacharyya, R. A. Kudgus, K. Giri, R. Bhattacharya and P. Mukherjee, Chem. Soc. Rev., 2012, 41, 2943–2970 RSC.
- S. Nie and S. R. Emory, Science, 1997, 275, 1102–1106 CrossRef CAS PubMed.
- W. Wang and S. A. Asher, J. Am. Chem. Soc., 2001, 123, 12528–12535 CrossRef CAS PubMed.
- J. Dai and M. L. Bruening, Nano Lett., 2002, 2, 497–501 CrossRef CAS.
- J. Zhang and J. R. Lakowicz, J. Phys. Chem. B, 2005, 109, 8701–8706 CrossRef CAS PubMed.
- C. Sonnichsen, B. M. Reinhard, J. Liphardt and A. P. Alivisatos, Nat. Biotechnol., 2005, 23, 741–745 CrossRef PubMed.
- Y. Zhang, K. Zhang and H. Ma, Anal. Biochem., 2009, 387, 13–19 CrossRef CAS PubMed.
- R. Shankar, L. Groven, A. Amert, K. W. Whites and J. J. Kellar, J. Mater. Chem., 2011, 21, 10871–10877 RSC.
- Z. Zhang, M. Li, Z. Wu and W. Li, Nanotechnology, 2011, 22, 015602 CrossRef PubMed.
- J. Yang, H. Yin, J. Jia and Y. Wei, Langmuir, 2011, 27, 5047–5053 CrossRef CAS PubMed.
- T. Soejima, S. Oshiro, Y. Nakatsuji and S. Ito, J. Colloid Interface Sci., 2011, 362, 325–329 CrossRef CAS PubMed.
- S. He, J. Yao, P. Jiang, D. Shi, H. Zhang, S. Xie, S. Pang and H. Gao, Langmuir, 2001, 17, 1571–1575 CrossRef CAS.
- S. L. Smitha, K. M. Nissamudeen, D. Philip and K. G. Gopchandran, Spectrochim. Acta, Part A, 2008, 71, 186–190 CrossRef CAS PubMed.
- Y. Chaikin, T. A. Bendikov, H. Cohen, A. Vaskevich and I. Rubinstein, J. Mater. Chem. C, 2013, 1, 3573–3583 RSC.
- Y. Sun, B. Mayers, T. Herricks and Y. Xia, Nano Lett., 2003, 3, 955–960 CrossRef CAS.
- S. Chen and D. L. Carroll, J. Phys. Chem. B, 2004, 108, 5500–5506 CrossRef CAS.
- S. H. Im, Y. T. Lee, B. Wiley and Y. Xia, Angew. Chem., Int. Ed., 2005, 117, 2192–2195 CrossRef.
- M. M. Oliveira, D. Ugarte, D. Zanchet and A. J. G. Zarbin, J. Colloid Interface Sci., 2005, 292, 429–435 CrossRef CAS PubMed.
- J. Zeng, Y. Zheng, M. Rycenga, J. Tao, Z.-Y. Li, Q. Zhang, Y. Zhu and Y. Xia, J. Am. Chem. Soc., 2010, 132, 8552–8553 CrossRef CAS PubMed.
- R. Sardar, J.-W. Park and J. S. Shumaker-Parry, Langmuir, 2007, 23, 11883–11889 CrossRef CAS PubMed.
- K. Kim, H. B. Lee, J. W. Lee and K. S. Shin, J. Colloid Interface Sci., 2010, 345, 103–108 CrossRef CAS PubMed.
- A. M. Signori, K. D. O. Santos, R. Eising, B. L. Albuquerque, F. C. Giacomelli and J. B. Domingos, Langmuir, 2010, 26, 17772–17779 CrossRef CAS PubMed.
- S. Tan, M. Erol, A. Attygalle, H. Du and S. Sukhishvili, Langmuir, 2007, 23, 9836–9843 CrossRef CAS PubMed.
- M. Liong, B. France, K. A. Bradley and J. I. Zink, Adv. Mater., 2009, 21, 1684–1689 CrossRef CAS.
- R. Kim, H. S. Park, T. Yu, J. Yi and W.-S. Kim, Chem. Phys. Lett., 2013, 575, 71–75 CrossRef CAS PubMed.
- T. Yu, R. Kim, H. Park, J. Yi and W.-S. Kim, Chem. Phys. Lett., 2014, 592, 265–271 CrossRef CAS PubMed.
- S. Y. Kang and K. Kim, Langmuir, 1998, 14, 226–230 CrossRef CAS.
- P. Vasileva, B. Donkova, I. Karadjova and C. Dushkin, Colloids Surf., A, 2011, 382, 203–210 CrossRef CAS PubMed.
- S.-B. Seo, J. Yang, E.-S. Lee, Y. Jung, K. Kim, S.-Y. Lee, D. Kim, J.-S. Suh, Y.-M. Huh and S. Haam, J. Mater. Chem., 2008, 18, 4402–4407 RSC.
- A. Köth, J. Koetz, D. Appelhans and B. Voit, Colloid Polym. Sci., 2008, 286, 1317–1327 Search PubMed.
- B. Bouchet-Fabre, K. Zellama, C. Godet, D. Ballutaud and T. Minéa, Thin Solid Films, 2005, 482, 156–166 CrossRef CAS PubMed.
- A. Henglein, Chem. Rev., 1989, 89, 1861–1873 CrossRef CAS.
- X. Huang, A. B. Schubert, J. D. Chrisman and N. S. Zacharia, Langmuir, 2013, 29, 12959–12968 CrossRef CAS PubMed.
- H. Zheng, R. K. Smith, Y.-W. Jun, C. Kisielowski, U. Dahmen and A. P. Alivisatos, Science, 2009, 324, 1309–1312 CrossRef CAS PubMed.
- T. H. Lim, D. McCarthy, S. C. Hendy, K. J. Stevens, S. A. Brown and R. D. Tilley, ACS Nano, 2009, 3, 3809–3813 CrossRef CAS PubMed.
- B. Lim, T. Yu, J. Park, Y. Zheng and Y. Xia, Angew. Chem., Int. Ed., 2011, 50, 6052–6055 CrossRef CAS PubMed.
- Y. Yin, Z.-Y. Li, Z. Zhong, B. Gates, Y. Xia and S. Venkateswaran, J. Mater. Chem., 2002, 12, 522–527 RSC.
- D. T. Sawyer and J. L. Roberts Jr, Acc. Chem. Res., 1988, 21, 469–476 CrossRef CAS.
- M. H. Rashid and T. K. Mandal, J. Phys. Chem. C, 2007, 111, 16750–16760 CAS.
- Q. Geng and J. Du, RSC Adv., 2014, 4, 16425–16428 RSC.
- S. Tang, S. Vongehr and X. Meng, J. Phys. Chem. C, 2010, 114, 977–982 CAS.
- P. Zhang, C. Shao, Z. Zhang, M. Zhang, J. Mu, Z. Guo and Y. Liu, Nanoscale, 2011, 3, 3357 RSC.
- Y. Chi, Q. Yuan, Y. Li, J. Tu, L. Zhao, N. Li and X. Li, J. Colloid Interface Sci., 2012, 383, 96–102 CrossRef CAS PubMed.
- Q. An, M. Yu, Y. Zhang, W. Ma, J. Guo and C. wang, J. Phys. Chem. C, 2012, 116, 22432–22440 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional TEM images, XPS spectra, TGA data, and UV-vis spectra of Ag nanoparticles. See DOI: 10.1039/c5ra00610d |
| This journal is © The Royal Society of Chemistry 2015 |