Recent advances in new generation dye removal technologies: novel search for approaches to reprocess wastewater
Akil Ahmad
a,
Siti Hamidah Mohd-Setapar
*ab,
Chuo Sing Chuong
a,
Asma Khatoona,
Waseem A. Wanic,
Rajeev Kumard and
Mohd Rafatullah
*e
aCentre of Lipids Engineering and Applied Research (CLEAR), Universiti Teknologi Malaysia, 81310 UTM Skudai, Johor, Malaysia. E-mail: sitihamidah@cheme.utm.my; Fax: +60 75581463; Tel: +60 75535496
bFaculty of Chemical Engineering, Universiti Teknologi Malaysia, 81310 UTM Skudai, Johor, Malaysia
cInstitute of Bioproduct Development, Universiti Teknologi Malaysia, 81310 UTM Skudai, Johor, Malaysia
dDepartment of Environmental Sciences, Faculty of Meteorology, Environment and Arid Land Agriculture, King Abdulaziz University, Jeddah 21589, Saudi Arabia
eSchool of Industrial Technology, Universiti Sains Malaysia, Penang 11800, Malaysia. E-mail: mohd_rafatullah@yahoo.co.in; mrafatullah@usm.my; Fax: +60 46573678; Tel: +60 46532111
First published on 12th March 2015
Abstract
Dyes are an important class of organic pollutants and are well known for their hazardous effects on aquatic life in general and human beings in particular. In order to reduce the negative effects of dye contaminated wastewater on humans and the environment, the wastewater must be treated carefully before discharge into main streams. Advances in science and technology have led to the evolution of several techniques for the removal of dyes from industrial and domestic effluents. In this review, the more recent methods for the removal of dyes from water and wastewater have been discussed. Wastewater treatment techniques such as adsorption, oxidation, flocculation–coagulation, membrane filtration and biological treatment have been highlighted. In addition, efforts were made to review all the available techniques and recently published studies from 2010–2014. Furthermore, the performance and special features of these technologies have been summarised. Advantages and limitations of each technique are also presented. A thorough literature survey revealed that chemical oxidation, adsorption, and biological treatments have been the most frequently investigated techniques for dye removal over the past few years.
1. Introduction
With the growing rate of industrialization, the emission of effluents from different industries poses serious threats to several living forms due to their adverse effects. Following the development of organic chemistry, and Perkin’s discovery of Mauveine in late nineteenth century, synthetic dyes emerged and grew rapidly. Within 50 years of Perkin’s discovery, synthetic dyes formed more than 90% of all the dyes in use.1 Nowadays, over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 types of dye are being manufactured according to the Colour Index, and annual worldwide dye production is more than 700
000 types of dye are being manufactured according to the Colour Index, and annual worldwide dye production is more than 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes.2 Commercially, azo dyes are the most important class of dyestuff owing to their superior tinctorial strength, easy preparation, cheap and easy availability of raw materials, ability to cover the whole shade range, and good fastness properties. Other classes of dye include anthraquinones, phthalocyanines, aryl-carboniums, and polymethines.
000 tonnes.2 Commercially, azo dyes are the most important class of dyestuff owing to their superior tinctorial strength, easy preparation, cheap and easy availability of raw materials, ability to cover the whole shade range, and good fastness properties. Other classes of dye include anthraquinones, phthalocyanines, aryl-carboniums, and polymethines.
Dyes find considerable application in several industries including textile, paper, plastic, rubber, concrete and medicine, with the textile industry as the main consumer of dyes. It is quite annoying to know that around 10% of dyes used in industry are discharged into the environment,3 which is quite harmful to the environment. Dye dissemination into water bodies leads to coloured water, which is a visible public concern. These dispersed dye molecules block sunlight from reaching the bulk of the affected water system, and therefore, reduce the dissolved oxygen (DO) level in the water. Dyes may also increase the biochemical oxygen demand (BOD) of the contaminated water body.
The toxicity level of a particular dye is very important due to its diverse effects on the environment and living organisms. The study of the harmful effects of dye constituents and their metabolites is very important for the establishment of strategies to reduce their acute toxic effects.4 Whilst some of the dyes do not possess significant acute toxicity, several dyes, particularly azo dyes, are known to be carcinogenic. Precisely speaking, azo dyes produce aromatic amines, which are highly toxic, carcinogenic or even explosive after the reductive cleavage of the azo group. Most common carcinogens such as benzidine are present in most of the dyes, which must be treated before their discharge into the environment.5 Besides dyes, other contaminants such as metals and auxiliaries used for dye manufacturing may be included. A study regarding the estrogenic and anti-estrogenic activity of textile dyes was reported recently,3 which further supported the harmful impacts of dyes to living organisms.
In order to control the negative impacts of dyes on living organisms, several techniques and methodologies have been developed for their removal from industry effluents and other water bodies. Briefly, dye removal from wastewater can be achieved through physical separation, chemical processes or biological degradation. Some important techniques which are widely used for the removal of dyes include adsorption, oxidation, biological treatment, electrochemical treatment, membrane filtration and coagulation–flocculation. Each technology has some merits and demerits. All kinds of wastewater containing dyes cannot be treated with one technology. Whether a technology may or may not be capable of the treatment of dye bearing water depends on the nature of the dyes, impurities and the composition of the wastewater. There are three types of dyes, anionic, cationic and non-ionic, which have different chromophoric and auxochromic groups.6 Anionic dyes are highly water soluble and difficult to remove by conventional methods. Biological treatments are not sufficient for the complete removal of acidic and reactive dyes.7 Nonionic dyes, also known as disperse dyes, do not ionize in an aqueous solution and their fused aromatic ring structure makes them highly resistant to degradation.6 However, a few cationic dyes like methyl blue can be easily removed by adsorption and advanced oxidation processes.
The present review article describes all these techniques for the removal of dyes from water and wastewater. The main aim of this review is to provide a detailed summary with the latest literature on different methods used for the removal of various dyes from industrial effluents.
2. Review background
Water stands as the second most important abiotic component of the ecosystem for living organisms. Geometrical population growth, modernization of civilizations, extensive industrialization, domestic and agricultural activities, and several geological and environmental changes are steadily poisoning the water quality of natural water resources.8–10 As a consequence, water pollution due to several natural and man-made activities is a serious problem in the present era and, therefore, government authorities, scientists, and academicians are continuously making efforts to reduce or fully eradicate this problem. Different technologies useful for the removal of dyes from water are available and several others are being developed. A thorough search of the literature on SciFinder and Google indicated approximately 950 research papers on the remedial technologies for the removal of harmful dyes and dye products from water. There has been a steady increase in the interest in research work being carried out in this direction, which is evident from the increase in the annual number of research papers that have appeared on this issue since 2010 (Fig. 1). In addition, several review papers have also been published, addressing the removal of different types of dyes from water using low cost adsorbants,11–14 activated carbon,15 nano-adsorbants,16,17 coagulation–flocculation18 and other methods.19–22 However, no review article was found addressing the advancements in all the techniques used for the removal of dyes from wastewater. Therefore, it was considered worthwhile to write this review and address the advancements in the removal of dyes from wastewater using activated carbon, nanoparticles, some low cost adsorbents, ion exchange, chemical precipitation, chemical coagulation–flocculation, oxidation, ozonation, photocatalytic degradation, electrochemical treatment, membrane filtration, ultrafiltration, nanofiltration, biological treatment, reverse micelle extraction and combined techniques. In addition, efforts have been made to discuss the current challenges faced in the development of technologies for the effective removal of dyes from wastewater and the future perspectives for the development of cheap, safe, non-toxic and effective removal technologies for water reprocessing. One of the main objectives in the writing of this article is to organise the scattered information on various dye contaminated water treatment technologies. We hope this article will serve as a useful reference material for beginners and other researchers actively involved in the development of technologies for the effective treatment of dye contaminated wastewater.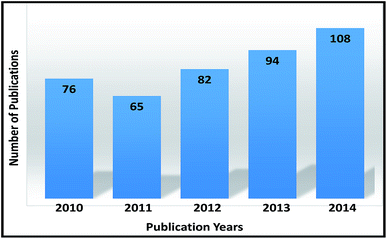 | ||
| Fig. 1 A pictorial representation of the increasing interest (except for 2011) in the development of treatment technologies for dye contaminated waters. | ||
3. Dye removal techniques
Over the last few decades, different physical, chemical and biological techniques have been developed to remove toxic dyes from wastewater and water reservoirs. Among all the techniques of dye removal, the adsorption process is the best choice for the decolourization of dyes and gives the best results for removal of various types of dissolved colouring materials.23,243.1. Adsorption
Adsorption is a surface phenomenon in which adsorbate molecules or ions (liquid or gas) are concentrated on the surface of a solid (adsorbent). The process can be classified as physisorption or chemisorption depending on how the adsorbate species are adsorbed onto the adsorbent surface.11 In the adsorption process, dyes molecules may be adsorbed on the surface of an adsorbent through several forces such as hydrogen bonding, electrostatic interactions, van der Waals forces, hydrophobic interactions etc.25 Generally, adsorbents possess porous structures to increase the total exposed surface area and allow fluid to pass through faster. Adsorption is a simple and economical method for dye removal from water and wastewater.26 Generally, adsorption has a high treatment efficiency and adsorbents can be regenerated for multiple reuses. The initial dye concentration, solution pH, temperature, contact time and adsorbent dosage are usually the main factors that govern the performance of most of the adsorption process. The removal of dyes from water and wastewater is generally carried out by adsorption using activated carbon, nanoparticulate adsorbents, low cost adsorbents and other types of adsorbents.In order to increase the adsorption capacity of AC, chemical and physical methods have been used for the development and modification of activated carbons. Physical activation is generally performed by the use of CO2, steam and other inert gases to remove the non-carbonaceous elements and open new pores at high temperature, while in chemical activation, organic or inorganic chemicals are used to modify and enhance the adsorption capacity of the AC. For chemical activation, generally a low temperature is used in the presence of a chemical which interacts with the carbon skeleton.27 Fernandez et al.28 developed activated carbon from orange peel biomass through the activation of H3PO4 acid and successfully applied this material for the removal of basic dyes, namely methylene blue and Rhodamine B, from aqueous media. The activated biomass material was applied in both batch and dynamic modes and showed a high adsorption capacity for both dyes. Mezohegyi et al.29 have demonstrated the role of activated carbon in removing dyes from an aqueous medium. They found activated carbon as a very economical and versatile material in decolouration. Njoku et al.30 used a novel agricultural waste, rambutan (Nephelium lappaceum) peel and prepared adsorbents in the form of activated carbon by chemically assisted KOH activation. The developed material was used for the removal of Acid Yellow 17 dyes and results indicated a high adsorption capacity, even at high initial dye concentrations, and the best isotherm fitted was the Langmuir isotherm model. The maximum monolayer adsorption capacity was reported as 215.05 mg g−1. Emami and Azizian31 applied date sphate as precursor to produce activated carbon by using phosphoric acid as an activating agent and the prepared material was successfully applied for the removal of methyl orange from aqueous solution. In the reported article, microwave irradiation was used in the activation process instead of furnace heating, causing a decrease in the operation time and a saving and homogeneous heating of the sample. Trevino-Cordero et al.32 successfully produced activated carbon from biomass of plum kernel and jacaranda. Activated carbon from plum kernel exhibited better removal of Acid Blue 25 and methylene blue. Besides, the presence of calcium salts on the surface of activated carbon greatly influenced the adsorption of dyes. Whilst this kind of research opens new possibilities for low cost production of activated carbon from agricultural by-products, further studies are required because the activated carbon produced may possess different properties to that produced from conventional raw materials. Besides biomass, activated carbon can also be produced from industrial wastes such as waste rubber tyres.33 The activated carbon from waste rubber tyres may be cost effective, efficient, and rapid for treating dye wastewater. Recently, Hadi and co-workers34 published a critical review on the preparation, characterization and wastewater treatment application of activated carbon derived from sludge. They observed that activated carbons produced by the chemical activation method showed superior adsorption capacity compared to the physically activated carbons. The adsorption capacity of the sewage sludge derived activated carbon not only depends on the texture properties but also the surface charge and functional groups present on the surface of the adsorbent. The activated carbon prepared from paper mill sewage sludge by steam activation was used as an adsorbent for the removal of methylene blue and Reactive Red 24 in a column process35 and it was observed that the prepared carbon showed better adsorption than commercial activated carbon and the sludge activated carbon showed a lower adsorption of negatively charged Reactive Red 24 dye (15.68 mg g−1) compared to cationic methyl blue (103.58 mg g−1).
| Magnetic nanoparticles | Dyes | Qm (mg g−1) | Conditions | Reference (s) |
|---|---|---|---|---|
| Magnetite nanoparticles | Methylene blue | 70.4 | Initial dye concentration: 1.6–32 mg L−1, pH: 9.2, dose: 0.3–0.9 g L−1, desorption: 85% | 38 |
| Congo Red | 172.4 | Initial dye concentration: 10.45–55.72 mg L−1, pH: 6.2, dose: 0.3–0.9 g L−1, desorption: 95% | ||
| N-Benzyl-O-carboxymethylchitosan magnetic nanoparticles | Methylene blue | 223.58 | Initial dye concentration: 100–300 mg L−1, pH: 3–5 | 39 |
| Crystal violet | 248.42 | |||
| Malachite green | 144.79 | |||
| Magnetic nanoparticles coated on activated maize cob powder | Methylene blue | 70.29 | Initial dye concentration: 250 mg L−1, pH: 6, dose: 0.4 g per 100 mL, 45 min | 40 |
| SDS modified magnetite nanoparticles | Safranin O | 769.23 | pH: 3, desorption: >95% | 41 |
| Magnetic B-cyclodextrin-chitosan/graphene oxide (MCCG) | Methylene blue | 84.32 | pH: alkaline, dose: 0.01 g per 25 mL | 42 |
| Organo-functionalized magnetite microsphere | Solvent Green 7 | 81.82–100.52 | Initial dye concentration: 70–90 mg L−1, pH: acidic | 43 |
| Magnetic ferrite nanoparticles–alginate composite | Basic Blue 9 | 106 | Initial dye concentration: 50 mg L−1, pH: 8 | 44 |
| Basic Blue 41 | 25 | |||
| Basic Red 18 | 56 | |||
| Palm kernel shell coated with iron oxide nanoparticles | Rhodamine B | 625 | Initial dye concentration: 100–500 mg L−1, pH: 5–8, adsorbent dose: 0.1 g | 45 |
| SDS modified magnetite nanoparticles | Methylene blue and Congo Red | 70.4 and 172.4 | Initial dye concentration: 30 mg L−1, pH: 6.2, 2 min, dose: 0.15 g L−1, desorption: 95% | 38 |
Among the various studies described in Table 1, Giri et al.38 use iron ore tailings, a waste from the steel and iron industry, to synthesize magnetite nanoparticles, providing a chance to re-use waste iron ore tailings. Acid leaching–precipitation and co-precipitation processes were used to produce magnetite nanoparticles. The synthesized magnetite nanoparticles showed rapid adsorption of methylene blue and Congo Red dyes. More than 85% desorption was achieved for both dyes, which indicated the reusability of the adsorbent. This work was indicative of the large scale operation of this methodology. Recently, Mahmoodi46 synthesized manganese ferrite nanoparticles by using manganese nitrate and iron nitrate and used it for the removal of dyes (Acid Red 18, Direct Green 6 and Direct Red 31) from a binary system. From the results, no selectivity was observed for the removal of dyes from the binary system using the magnetic adsorbents.
Debrassi et al.39 used chitosan derivatives in their magnetic nanoparticles to remove methylene blue, crystal violet, and malachite green with adsorption capacities of 223.58, 248.42 and 144.79 mg g−1, respectively. The adsorbent was tested for three repeated cycles with negligible effect on adsorption performance indicating the high adsorption potential of the nanoparticulate system. Fan et al.42 pooled several substrates, including chitosan, in their adsorbent. The resulting adsorbent possessed the features of its individual constituents such as higher stability and adsorption capacity, and easy separation. The adsorbent was very efficient in removing methylene blue.
In addition to magnetic nanoparticles, some non-magnetic nanoparticles have also been reported for the removal of dyes from water. Ahmed et al.47 investigated nano-polyaniline to remove Acid Red 14. The adsorption capacity of the adsorbent was 323 mg g−1, which was reported to improve to 430 mg g−1 by adding baker’s yeast, indicating the potential of a polyaniline nanoparticulate system with a good tuning range as an adsorbent of choice. Lee et al.48 demonstrated the use of nano-sized aminopropyl functionalized magnesium phyllosilicate (AMP) clay for the removal of malachite green. The maximum adsorption capacity was 334.8 mg g−1 with 81.72% dye removal with 0.1 mg mL−1 AMP clay added, and the authors envisaged that complete dye removal can be realized at above 0.2 mg mL−1 AMP clay. Later, Assefi et al.49 synthesized a cobalt(III) oxide (Co2O3) nanoparticle loaded on activated carbon and it was observed that this is an outstanding sorbent for the removal of eosin Y (EY) as a hazardous dye from aqueous solution. From the results, it was concluded that Co2O3-NP–AC can be used as an efficient, green and low-cost adsorbent for the removal of dyes from aqueous solutions, having high adsorption capacity.
A low-cost adsorbent, castor bean (Ricinus communis L.) press cake, a by-product from the biodiesel production process, was used for the adsorption process by Magriotis et al.50 and applied to the removal of malachite green (MG) and tropaeolin (TP) dyes from aqueous solutions. The results are quite promising, therefore it was confirmed that castor bean press cake is an alternative low cost adsorbent for the removal of dyes from aqueous solutions, since it is effective and available in large amounts.
Ahmaruzzaman and Gupta51 reviewed the application of rice husk and its ash as low cost adsorbents for treating various pollutants and demonstrated that rice husk and its ash have good potential for the removal of various pollutants from water and wastewater. Salleh et al.52 also reviewed the use of agricultural solid wastes as adsorbents for the removal of dyes. Others investigated the potential of chitosan,53 timber sawdust,54 coffee residues,26 milled sugarcane bagasse,55 modified palm empty fruit bunch,56 magnetite nanoparticles loaded tea waste,57 dried prickly pear cactus cladodes,58 treated citrus biomass,59 treated wheat straw60 and Jania adhaerens biomass.61 Some of their adsorption results are given in Table 2.
| Low cost adsorbents | Dyes | Qm (mg g−1) | Conditions | Reference (s) |
|---|---|---|---|---|
| Alkaline treated timber sawdust | Methylene blue | 1928.31 | Initial dye concentration: 150 mg L−1, dose: 1 g L−1, 10 min | 54 |
| Methyl green | 1821.33 | |||
| Coffee residues | Remazol Blue RN | 179 | pH: 2, 10 respectively, dose: 1 g L−1 | 26 |
| Basic Blue 3 G | 295 | |||
| Milled sugarcane bagasse | Rhodamine B | 65.5 | Initial dye concentration: 250 mg L−1, dose: 1 g L−1 | 55 |
| Basic Blue 9 | 30.7 | |||
| Modified palm EFB fibre | Methylene blue | 130 | pH: 7, citric acid modified | 56 |
| Phenol red | 171 | pH: 3, PEI modified | ||
| Dried prickly pear cactus cladodes | Methylene blue | 189.83 | pH: acidic, dose: 1 g L−1 | 58 |
| Eriochrome Black T | 200.22 | pH: alkaline, dose: 3 g L−1 | ||
| Alizarin S | 118.35 | |||
| Treated wheat straw | Methyl orange | 300 | Initial dye concentration: 1000 mg L−1, pH: low, dose: 1 g L−1, 48 h | 60 |
| Acid Green 25 | 950 | |||
| Methylene blue | 100–130 | Initial dye concentration: 500 mg L−1, pH: <10, dose: 1 g L−1, 48 h | ||
| HCl treated Jania adhaerens biomass | Acid Blue 25 | 95.40% removal | Initial dye concentration: 20 mg L−1, pH: 2, dose: 1.2 g L−1 | 61 |
| Sulphuric acid and zinc chloride treated ginger waste | Crystal violet | 277.7 | Initial dye concentration: 5–20 mg L−1, pH: 9, adsorbent dose: 0.05 g, 180 min | 62 |
| Oxidized cactus fruit peel | Brilliant Green | 166.66 | Initial dye concentration: 200–500 mg L−1, pH: 3, adsorbent dose: 0.025 g, 240 min | 63 |
| Treated bagasse | Methylene blue (MB) and malachite green (MG) | 69.93 and 65.79 | Initial dye concentration: 100 mg L−1–300 mg L−1, pH: 2–10, adsorbent dose: 0.2–1.0 g, temperatures 27 ± 1–60 °C | 64 |
| Mesoporous aluminophosphate | Methylene blue (MB) and malachite green (MG) | 35.2 and 24.51 | Initial dye concentration: 100–500 mg L−1, pH: 10, thermal stability 1173 K, 20–30 min | 65 |
| Chemically modified brown macroalga | Acid Orange II (AO7) | 45.47 | Initial dye concentration: 30–90 mg L−1, pH: 2, 60 min, biomass dose: 0.2–2.2 g L−1 | 66 |
| Amberlite IRA-958 | Acid Orange 7 | 50 | Initial dye concentration: 50–500 mg L−1, pH: 2–12, adsorbent dose: 0.2 g, 180 min, desorption: <50% | 67 |
Biomass may itself exhibit high adsorption of dyes but it can be greatly enhanced by suitable treatments. For instance, the adsorption capacities of timber sawdust for methylene blue and methyl green are 694.44 and 892.86 mg g−1.54 After alkaline treatment, the adsorption capacities increase to 1928.31 mg g−1 and 1821.33 mg g−1 for methylene blue and methyl green, respectively. The equilibrium time and re-usability is also greatly improved after alkaline treatment. Wen et al.68 studied the potential of glow discharge plasma (GDP) to enhance the dye adsorption performance of chitosan. The adsorption capacity for Acid Red 73 was 69.54 mg g−1 for untreated chitosan which increased to 99.80 mg g−1 by modifying chitosan using 50 mA GDP. Modifying chitosan using 120 mA GDP gave an adsorption capacity of 121.8 mg g−1. The modified chitosan was tested for 10 other dyes and all showed significant improvement in dye removal percentages compared to untreated chitosan.
Besides agricultural biomass, algal biomass can also be used as an adsorbent for dye removal. Kousha et al.61 tested the potential of red algae, Jania adhaerens, to remove Acid Blue 25. The results showed that HCl treated J. adhaerens biomass had the highest percentage of dye removal (95.4%) compared to untreated J. adhaerens biomass (49.41%) and methanol treated J. adhaerens biomass (58.18%) at optimized conditions. Kousha et al.66 investigated the use of brown microalgae, Stoechospermum marginatum, to remove Acid Orange II. Various types of pre-treatments were applied to the brown microalgae to study their effects on dye adsorption. Esterification, formaldehyde pre-treatment, and methylation were found to reduce the adsorption efficiency. It was concluded that propylamination greatly improved the dye adsorption capacity from 35.62 to 71.05 mg g−1 after treatment.
Meziti and Boukerroui69 reported the use of spent bleaching earth (SBE) as an adsorbent to remove Basic Red 46. The SBE used in this investigation was obtained from the waste from edible oil refining. It was pre-treated through NH4Cl solution, heat treatment, and washing with HCl before used as an adsorbent. The adsorption capacity of regenerated SBE is 73 mg g−1 while virgin bleaching earth had an adsorption capacity of 84.03 mg g−1. This report provides a clue to the usage of an another alternative low cost adsorbent.
It is quite evident from the discussion in the above section and the data from Table 2 that low cost adsorbents serve efficiently in the removal of toxic dyes from wastewater. Their low cost, efficient regeneration, easy preparation and eco-friendly nature are quite encouraging to stimulate further research into the development of water treatment technologies using low cost adsorbents so that a few usable systems in future may be obtained.
Other recent studies included the use of TiO2/Ag modified penta-bismuth hepta-oxide nitrate to remove methyl orange and Sunset Yellow,73 mesoporous carbon CMK3 to remove methyl orange,74 and mesoporous aluminophosphate to remove malachite green and methylene blue with high removal percentages of 94% and 98%, respectively, within only 20 min.65 Mesoporous aluminophosphate had a high thermal stability, high porosity, environmental friendly and safe manufacturing process, and high potential for regeneration (99% and 99.5% for malachite green and methylene blue).
3.2. Ion exchange
The ion exchange process is one of the most common techniques, which can effectively remove dyes from aqueous solutions through strong interactions between charged dyes and functional groups on ion exchange resins. This process involves the exchange of ions to form strong bonds between solutes and resins, thereby achieving effective separation.75 The resins are available as anion exchangers or cation exchangers for separating solutes with different surface charges. Greluk and Hubicki76 extended their studies towards two Acid Orange dye removal strategies. They investigated the efficiency of two strongly basic anion exchangers and one weakly basic anion exchanger to remove Acid Orange 7 and Acid Orange 10 from water. The presence of a sulfonic group on the acid dyes was thought responsible for the ion exchange process. Besides, the dye molecules with more sulfonic groups displayed enhanced and faster binding on all types of anion exchangers; in addition, the basicity of the resins was proved to have significant effects on the adsorption capacity of dyes. Greluk and Hubicki67 studied the removal of Acid Orange 7 using a strongly basic ion exchange resin. The authors observed that variations in pH, temperature, salt concentrations, and the presence of anionic and non-ionic surfactants had insignificant effects on dye removal. However, the presence of cationic surfactants greatly reduced the removal efficiency due to dye–surfactant interactions that hindered adsorption of Acid Orange 7 on the resin. At optimized conditions, the resin can remove up to 99% Acid Orange 7 in 10 min. However, the difficulty in desorption was the major drawback associated with this technique. Wawrzkiewicz77 studied the Direct Red 75 removal potential of weakly and strongly basic gel anion exchangers. The highest achievable desorption yield was 60%, and the process also showed difficulty in the desorption process.Recently, Wawrzkiewicz78 studied the effectiveness of cation exchange resins to remove Basic Blue 3. It was shown that 99.9% dye removal can be achieved in one hour of contact time. However, the presence of SDS in high concentrations greatly reduced the removal efficiency due to dye–surfactant interactions. Desorption of the cation exchange resin can reach to 100% in optimized conditions. Shuang et al.79 synthesized a novel quarternized magnetic resin NDMP to remove Orange G and Red RWO dyes. This resin worked well within a large pH range of 2–11. The equilibrium adsorption amounts on NDMP of Orange G and Red RWO were 1.9 and 0.7 mmol g−1, respectively. This amount was twice that of the magnetic ion exchange resin. NDMP also showed good desorption capability; almost 100% for Orange G and 90% for Red RWO. The resin was used for 20 cycles during this study with a slight decrease in adsorption efficiency.
Naturally occurring compounds can be used as ion exchange resins. Constantin et al.80 prepared an ion exchanger based on pullulan microspheres to remove Azocarmine B. The maximum adsorption capacity was 113.63 mg g−1. The beauty of pullulan lies in the fact of it being biodegradable and a low cost ion exchanger for dye removal applications. Alver and Metin81 modified natural zeolite and used it to remove Reactive Red 239 and Reactive Blue 250. It was interesting to note that 97% and more than 99% removal can be achieved for Reactive Red 239 and Reactive Blue 250, respectively, in only 30 min.
The reports in this sub-section indicate that ion exchange resins have been extensively investigated for their dye removal efficiencies from wastewater. Of course, scientifically significant results have been reported in some studies. Besides, these reports can provide a rational for how better systems can be developed. The factors affecting dye removal efficiency should be more keenly followed so that better systems can be developed to remove dyes from wastewater at pilot scale.
3.3. Chemical precipitation
Chemical precipitation is a relatively simple wastewater treatment technique in which chemicals such as sulphides, hydroxides and carbonates react with organic and inorganic pollutants present in wastewater to form insoluble precipitates. Chemicals react with dissolved dye molecules to form insoluble precipitates and then can be removed. The general procedures involve the addition of chemicals into wastewater treatment to form the precipitate with dye molecules and waiting for the insoluble particles to settle. Then the wastewater can be decanted to separate the sludge. The most common chemical precipitation method for dye removal is hydroxide precipitation. Bouyakoub et al.82 used magnesium chloride and manganese chloride to remove Levafix Brilliant Blue EBRA and found that most dyeing auxiliaries greatly increase the chemical dosage needed to remove the dye.Zhang et al.83 used the leaching solutions of white mud to remove Acid Orange II, Reactive Light Yellow K-6G, Reactive Bright Red K-2BP and Direct Yellow R. More than 90% dye removal was achieved within 90 s, which showed the fast kinetics of this system. This study also showed 94.6% colour removal for industrial effluents at 4 g L−1 sorbent. Besides, the treated water was further proven to be non-toxic. This report clearly indicated the potential of chemical precipitation for the large scale treatment of water for drinking and other purposes. Oladoja et al.84 synthesized CaCO3 and Ca(OH)2 from gastropod shells. In situ hybridization of methylene blue and Congo Red into growing particles of calcium derivatives was investigated. The precipitation of methylene blue and Congo Red was found to be around 67–77 and 98% respectively. In addition, the initial pH, initial dye concentration, presence of anions and ionic strength had negligible effects on dye removal. However, it was interesting to notice that anions produced a larger sludge volume while higher ionic strength increased the sludge settling rate. Chemical precipitation is an efficient method for the removal of organic dyes from wastewaters, but generation of sludge and high chemical cost are the major hurdle for the application of this technology at industrial scale.
3.4. Coagulation–flocculation
Coagulation and flocculation are an essential part of drinking water treatment as well as in wastewater treatment plants. The principle is to destabilize the dispersed solid particles in water by reducing their surface charge and gathering them to form larger particles. Its procedures can be divided into three steps: flash mixing, coagulation and flocculation. Firstly, coagulants are added with violent mixing. Then, the coagulants act to reduce or neutralize the surface charge of finely dispersed particles. After that, flocculants and gentle mixing are used to bring the fine particles close together and to form larger particles. Sedimentation is usually applied to remove the larger particles afterwards. Various types of coagulants and flocculants have been used for wastewater treatment. Sometimes coagulant aids are also added to enhance the treatment process. Lee et al.85 studied the significance of five parameters on Cibacron Red FN-R removal using an inorganic–organic composite polymer through a flocculation system. The dosage of composite polymer was the most significant factor for dye removal, followed by pH, dye concentration, agitation speed, and the least significant factor was agitation time. This report served as a stimulus for motivating and inspiring several other researchers to develop advanced and optimized systems based on coagulation–flocculation for the safe and efficacious treatment of wastewater.Coagulants can be metal salts, polymers or some naturally occurring materials. In recent years, researchers have used calcium chloride,86 aluminum sulphate,87 polymeric aluminum sulphate,86 polyaluminium chloride and polyaluminium chloride sludge,88 and other naturally occurring materials89 as coagulants to remove dyes from water. Table 3 gives an overview of different materials used in coagulation–flocculation procedures for the removal of dyes from wastewater.
| Coagulants and chemicals | Dyes | Removal (%) | Conditions | Reference (s) |
|---|---|---|---|---|
| Calcium chloride | Blue Bezaktiv S-GLD | 94 | Initial dye concentration: 40 mg L−1, pH: 5–9 | 86 |
| Polymeric aluminum sulphate | Black Novacron R | 93 | ||
| Aluminum sulphate | Acid Black 210 | 97.78 | Initial dye concentration: 4 g L−1, pH: 5.61, dose: 0.82 g L−1, 40 °C | 87 |
| Polyaluminium chloride | Acid Red 119 | 95.25 | Initial dye concentration: 140 mg L−1, pH: 3.8, dose: 57 mg L−1 | 88 |
| Polyaluminium chloride sludge | 94.10 | Initial dye concentration: 140 mg L−1, pH: 3.42, dose: 4.55 g | ||
| CaCO3 and Ca(OH)2 | Methylene blue (MB) or Congo Red | 67–77% | Initial dye concentration: 25–100 mg L−1, pH: 4–10, 30 min | 84 |
| Surjana seed powder, maize seed powder and chitosan | Congo Red | 98 | Initial dye concentration: 60 mg L−1, pH: 4, 60 min flocculation, dose: 25 mg L−1 | 89 |
| Leaching solution of white mud | Reactive Bright Red K-2BP, Reactive Light Yellow K-6G, Acid Orange II and Direct Yellow R | 99.6, 93.5, 97.5, and 98 | Initial dye concentration: 100 mg L−1, pH: 12, dose: 2 g L−1, 90 s, 20 °C | 83 |
Merzouk et al.90 compared the effectiveness of ferric chloride and aluminium sulphate to remove dispersed red dye. The results showed that aluminium sulphate was more effective than ferric chloride, which removed more than 90% color at a dosage of 40 mg L−1, pH range 4–8, and dye concentration up to 235 mg L−1. The presence of high salt content was found to reduce the removal slightly. The authors also concluded that chemical coagulation was more robust to pH change and had lower cost compared to electrocoagulation.
In order to reduce the dependence of water treatment procedures on synthetic coagulants, several naturally occurring coagulants have been investigated, which are biodegradable and quite safe to handle. Chitosan, surjana seed powder and maize seed powder were used by Patel and Vashi89 as coagulants to remove Congo Red. Dye removal at optimized conditions was 94.5% for chitosan, 98% for surjana seed powder and 89.4% for maize seed powder. Therefore, it is quite obvious that the use of naturally occurring coagulants is a step in the right direction for the removal of dyes from wastewater with lower cost and greater safety profiles.
Industrial wastes have been found to have considerable uses in water treatment processes. Thus, the utilization of these wastes will help to improve the environment at lower cost. Bittern is a by-product of solar salt production. The bittern wastewater studied by Ayoub et al.91 contains high concentrations of magnesium ions which can act as coagulants. The potential of bittern wastewater to remove Indigo Blue dye was studied by Albuquerque et al.92 From the results, the mechanism was found to be different for each coagulant. The bittern wastewater can be applied efficiently and economically as a coagulant in the physical–chemical treatment of alkaline textile effluents. Over 80% dye removal was reported by the authors. Coagulant aids such as sodium alginate were found to reduce the amount of aluminum sulphate needed to remove dyes.93 It also enhanced the process by giving larger flocs, higher floc strength and better recovery ability.
Flocculants are polymers, which enhance floc aggregation to form larger particles for easier separation. Some flocculants include sodium poly-methacrylate and CHT-flocculant CV.86 The COD removal and sludge production were also tested and acceptable results were found. Wang et al.94 developed a cationic organic flocculant, epichlorohydrin–dimethylamine (EPI–DMA), to remove Acid Cyanine 5R and Direct Violet N. The results showed 90% Acid Cyanine 5R (0.1 g L−1) removal with 10 mg L−1 EPI–DMA and more than 90% Direct Violet N (0.1 g L−1) removal with 6 mg L−1 EPI–DMA. The authors identified EPI–DMA viscosity and cationicity as important factors influencing properties of floc formed with acid dyes and direct dyes.
3.5. Oxidation
Oxidation is a very important method for the treatment of wastewater by using oxidizing agents. Mainly two forms of oxidation are reported such as chemical oxidation and UV assisted oxidation using chlorine, hydrogen peroxide, Fenton’s reagent, ozone, or potassium permanganate for treatment of industrial and wastewater effluents. In the oxidation process, pH and catalysts play an important role.| O3 → OH˙ + O2− + HO3˙ + HO4˙ |
| OH˙ + O2− + HO3˙ + HO4˙ + dyes → mineralized by-product |
The ozone is usually produced by passing air or oxygen through the gap between two discharging electrodes. The ozone is then infused into the wastewater for the treatment or disinfectant process. Agents such as peroxides, UV and conditions of high pH assist ozone in the oxidation process. The optimum pH can be in the acidic or alkaline range depending on the type of dyes in the wastewater. Although ozonation is proven to be very effective for decoloration, it may produce by-products, which are more toxic and hazardous than dye molecules. The by-products must also be degraded to ensure that there will be no harm to the environment. Often, ozonation itself is sufficient to degrade all the harmful by-products but it will take longer time and increase the operation cost. A combination of processes helps to reduce cost while achieving acceptable degradation. Mezzanotte et al.95 used high dose ozonation (up to 60 mg L−1) to complete the dye removal. The authors found that color removal can be used to predict the toxic potential of ozonation by-products such as glyoxal and methylglyoxal concentrations. Another advantage was no sludge production which greatly simplifies the operation.
Catalysts can be added to enhance ozonation process. Mahmoodi96 studied the photocatalytic ozonation of dyes with copper ferrite nanoparticles. Mineralization gives relatively safe compounds (NO3− and SO42−) using this method. An advantage of this method is the potential to treat high volumes of effluent without the use of high pressure oxygen or heating.
| (TiO2) + hν → h+VB + e−CB |
| e−CB + O2 → ˙O2 (reduction) |
| h+VB + H2O → ˙OH + H+ (oxidation) |
Gupta et al.99–101 studied UV/TiO2 for the degradation of hazardous Tropaeolin 000, tartrazine and quinoline yellow. The process was sensitive to solution pH and initial dye concentration, followed pseudo-first order kinetics, and complete mineralization was achieved. Karimi et al.102 reported the photocatalytic degradation of azo dyes using nano-strontium titanate and suggested that nano-strontium titanate is a very efficient photocatalyst for dye degradation. Comparisons were made between two photocatalysts, nano-strontium titanate and nano-titanate oxide, which have the same particle size, and it was demonstrated that nano-strontium titanate was more advanced and efficient in the photocatalyst oxidation process for Direct Green 6 and Reactive Orange72 dye degradation.
A novel composite silver nanoparticle–colemanite ore waste (Ag–COW) was synthesized by Yola et al.103 and its adsorption and photocatalytic behaviour towards Reactive Yellow 86 (RY86) and Reactive Red 2 (RR2) from an aqueous medium in single and binary systems was reported. From the results, it was observed that Ag–COW is a more effective material for dye removal from aqueous media with a combination of both adsorption as well as photocatalysis.
Yuan et al.104 studied the effect of chloride ions, as one of the main components in dye containing wastewater, on UV/TiO2 degradation of Acid Orange 7. The chloride ion had dual effects on dye removal. A low chloride ion concentration enhanced the removal but a high concentration inhibited the removal process. It was interesting to note that the inhibitory effect of the chloride ion was stronger at lower pH.
In order to increase the dye removal performance of photocatalytic degradation, researchers studied the modifications of a TiO2 photocatalyst and Pt-TNT, which was tested on seven azo dyes.105 Saleh and Gupta106 enhanced the efficiency of a UV/TiO2 system by incorporating a co-adsorbent, multi-walled carbon nanotubes (MWCNT). This MWCNT/TiO2 composite had better photocatalytic activity compared to TiO2 only due to the prevention of the recombination of photo-generated electron–hole pairs and a large surface active area. Yan et al.107 produced porous SnIn4S8 microspheres as photocatalysts to remove methyl orange, Rhodamine B and methylene blue with 95, 100 and 100% removal, respectively. Complete mineralization was achieved after 5 hours. Upadhyay et al.108 synthesized cadmium sulphide nanoparticles as photocatalysts to remove crystal violet and methylene blue with up to 96% and 87% removal, respectively, within 105 min. Li et al.109 proposed electrochemical oxidation as a pre-treatment before photocatalytic oxidation. Complete removal of methylene blue (100 mg L−1) was achieved in 4 hours. The authors mentioned this method as a highly efficient and energy saving way to treat high chroma methylene blue solution.
Hydrogen peroxide (H2O2) is a popular oxidizing agent for wastewater treatment. Many studies have been carried out using H2O2 as one of the main components to remove dyes from water. Some recent studies used CuO/γ-Al2O3/H2O2 to remove Amaranth dye (90% removal),110 cobalt tetrasulfophthalocyanine monomer encapsulated in mesoporous silica/H2O2 to remove Acid Red 73 (82% removal),111 Fe alginate gel beads/H2O2/UV to remove Reactive Blue 222 and Acid Black 234,112 nickel tetrasulfophthalocyanine encapsulated in silica/H2O2 to remove methyl orange (96% removal),113 and TiO2/UV-LED/H2O2 for complete removal of Rhodamine B.114 These studies showed that a wide range of catalysts are available to use with H2O2 for efficient removal of dyes. Some studies also showed the re-usability of catalysts.
Enzymes are being used in decoloration due to mild operating conditions and environmental friendliness. Zhang et al.115 used chloroperoxidase (CPO) as a catalyst for H2O2 oxidative degradation of Orange G and Sunset Yellow. CPO was extracted from Caldariomyces fumago. This CPO/H2O2 combination showed excellent performance of 98.72% Orange G removal in 5 min and 77.25% removal of Sunset Yellow in 10 min at pH 2.75. In a recent study, Liu and co-workers116 explored the mechanism of enzymatic degradation of alizarin red S and crystal violet dyes. During the CPO catalytic reaction a few small intermediate compounds having high oxidative activity, such as Cl2, HClO, and HOOSO2(OH)/HSO3OOSO3H, formed on the active surface of CPO. These species are mainly responsible for the degradation of the dye molecules/ions. Dulman et al.117 developed a new catalyst, Cu2+ adsorbed on macroporous chelating polymer, for H2O2 oxidative degradation of Orange G. This oxidation system was able to remove completely the Orange G within 30 min at 24 °C. Further increasing the temperature up to 50 °C reduced the reaction time (15 min) for complete removal of Orange G.
The performance of photodegradation, H2O2 degradation, Fenton oxidation process, and a combination of these processes for the removal of Reactive Blue 19 was compared by Guimaraes et al.118 The results showed that significant removal cannot be achieved with UV or H2O2 alone. Whilst UV/H2O2 can give 100% removal, it is slow and requires high doses, making it expensive. However, the UV/Fe2+/H2O2 process was found to be the most effective method to treat Reactive Blue 19. Generally, during the photodegradation, H2O2 degradation and Fenton oxidation processes, highly oxidizing species like hydroxyl radicals are produced, which are mainly responsible for the mineralization of the organic pollutants.
In oxidation degradation, the removal of dye using UV/H2O2 depends on some important factors such as peroxide concentration, treatment time, intensity of UV radiation, pH, chemical structure of the dye and additives. But the drawback of this process is the production of undesirable by-products and the expense for small scale industries. Sometime degradation products are more toxic than the parent one. Moreover, expose to the UV radiation is harmful and may cause skin problems. Although, the process has high potential and is technically sound for the treatment of coloring materials.
Other chemicals which were investigated for dye removal through oxidation include Co2+/PMS120 and the palladium/hydroxyapatite/Fe3O4 nanocatalyst.121 All of the mentioned systems give near complete color removal under tested conditions. However, some of them can only give partial mineralization.
Some processes can be incorporated in the oxidation system to enhance dye removal performance. Non-thermal plasma treatment was applied by Benetoli et al.122 to remove methylene blue from water. High decoloration was achieved in both studies although different feed gases and catalysts were used. However, the oxygen/pyrite pair gave better mineralization (70%) than the air/Fe2+ pair (21%).
Ultrasound can be used in advanced oxidation processes for dye degradation. Eren123 reviewed the role of ultrasound in oxidative degradation of dyes. Geng and Thagard124 applied ultrasound to degrade Rhodamine B. Effects of various parameters such as amplitude, external pressure, dye concentration and temperature on dye degradation were investigated. It was found that increasing amplitude and hydrostatic pressure increased dye removal; increasing temperature increased dye removal at lower amplitude but had an insignificant or negative impact at higher amplitude. Finally, increasing initial dye concentration was found to decrease dye removal.
Another oxidation system being studied for dye removal is catalytic wet air oxidation. Hua et al.125 used CuO/γ-Al2O3 as a catalyst for wet air oxidation of methyl orange, Direct Brown and Direct Green. Complete decoloration and 70% TOC removal were achieved in all cases in 2 hours under optimized conditions. Ovejero et al.126 used Ni/MgAlO as a catalyst for wet air oxidation of Basic Yellow 11. Up to 98% removal can be achieved and the catalyst was re-used three times with little drop in performance. Vallet et al.127 also used Ni/MgAlO as catalyst and studied the wet air oxidation of Chromotrope 2R in a trickle bed reactor. In situ catalyst regeneration was applied and this enabled long term operation (>24 hours) of the reactor with no loss of catalytic activity.
3.6. Electrochemical treatment
Over the past two decades, electrochemical treatments have regained their importance worldwide due to the increasing demand for fresh drinking water. Electrochemical treatments of dyes mainly include electrocoagulation and electrochemical oxidation, which have high efficiency, easy operation and require compact facilities.The main difference between electrocoagulation and chemical coagulation is that coagulants are generated from the anode during electrocoagulation and no secondary pollution occurs. Recently, several studies using electrocoagulation for the removal of dyes were conducted by Amani-Ghadim et al.128 The anodes used were mainly of iron and aluminum. It was quite interesting to note that more than 90% dye removal can be achieved in a short time. For example, more than 99% Reactive Red 43 was removed using an aluminum anode within 12 min.
The performance of electrochemical oxidation using various electrodes such as titanium, PTFE, iron, graphite and boron-doped diamond were studied by Senthilkumar et al.,129 Prakash et al.,130 Korbahti et al.,131 Rosales et al.132 and Vahid and Khataee133 (2013). An et al.134 synthesized a TiO2-NTs/Sb–SnO2/PbO2 anode for electrochemical degradation of Reactive Blue 194 and it gave more than 90% dye removal at optimized conditions.
Suitable equipment is required for the proper operation of electrochemical oxidation. An electrochemical cell was developed by Rivera et al.135 for anodic oxidation of Reactive Black 5. It was shown that a cylindrical cell is better than a cubic cell in terms of removal efficiency. Complete decoloration and 95% TOC removal were achieved by using this electrochemical cell in 3 hours. Pilot scale electrochemical oxidation of methyl orange was studied by Ramirez et al.136 A three litre flow plant was used for the study. After optimization through RSM, 95.9% decoloration and 60.3% TOC was achieved within 138 min. The authors mentioned that current density has a significant impact on dye removal.
Since the operating cost is a major concern for electrochemical treatment, research is deeply concerned with reducing the electricity usage. Kariyajjanavar et al.137 stated that increasing NaCl salt reduced the operating voltage. By incorporating an ultrasound technique, Siddique et al.138 successfully reduced the energy consumption of Reactive Blue 19 degradation to half compared to conventional electrochemical oxidation. Several studies were also carried out to enhance the performance of electrochemical oxidation. Riera-Torres and Gutiérrez-Bouzan139 applied UV treatment after electrochemical oxidation to enhance decoloration and the removal of halogenated compounds. From the results, more than 92% decoloration and good COD removal were reported. Somayajula et al.140 studied the effects of ultrasonic waves and electrolyte concentration on the removal of Reactive Red 195. It was found that higher sonic power lowers the dye removal. Dye removal in NaCl (99%) and KCl (99%) were also found to be much better compared to Na2SO4 (52%) and Na2CO3 (39%). Complete decoloration was achieved for 100 mg dye per L in 50.32 min through sono-electrochemical oxidation.
Electrochemical reduction is another type of electrochemical treatment. However, it is not widely used for dye removal. del Río et al.141 compared different types of electrochemical treatments to remove Reactive Orange 4 and found that electrochemical reduction has the slowest decoloration. On the other hand, electrochemical oxidation has the highest mineralization rate while electrochemical oxido-reduction produced highly oxidized intermediates. This technique is very effective for the removal of soluble and insoluble dyes. Beside other variables, dye removal also depends on the anode’s material and working potential. Consumption of high electricity, production of sludge and pollution from chlorinated organic materials are the main drawbacks of this technique.
3.7. Membrane filtration
Membrane filtration is an advanced treatment technology for the removal of colour, COD and salinity from wastewater.142 The procedure involves passing the wastewater through membranes with small pores. Any solutes which are larger than the pore size will be trapped and the solution, after passing through the membranes, is free from those solutes. The trapped solutes form a layer of filter cake and must be removed constantly to ensure the smooth running of the filtration process. Membrane filtration can be classified based on the size of the pores on the membranes. The performance of membranes is usually evaluated through the rejection and permeates flux.Since ultrafiltration membranes alone may not be enough to ensure acceptable dye removal at reasonable operating conditions, additives can be used to improve rejection. Dong et al.145 used powdered activated carbon (PAC) to improve dye rejection by adsorption. The results showed significant improvement from 43.6% rejection with the ultrafiltration membrane alone to near 100% rejection in 20 min at 100 kPa trans-membrane pressure when PAC was deposited on the membrane surface. Increasing the PAC amount and operating pressure would reduce the time needed for complete decoloration.
Polyelectrolyte enhanced ultrafiltration (PEUF) was studied by Mondal et al.146 In PEUF, polymer molecules undergo complexation with solutes to form macromolecules which can be easily retained by the ultrafiltration membrane. The polymer poly(acrylic acid), poly(ammonium acrylate) and cellulose membranes (10 kDa) were used. The results showed a significant increase in rejection when polymer concentration was increased. Variations in pH also affected the dye–polymer interactions. High removal was achieved at 2 bar trans-membrane pressure.
Another method to enhance ultrafiltration involves the addition of surfactants into the dye solution. This method is called micellar enhanced ultrafiltration (MEUF). The surfactant molecules form micelles, trapping charged dye molecules in them. This allows the membrane to retain both the dyes and surfactants easily. Ngang et al.147 studied the MEUF of methylene blue with SDS as a surfactant using a polysulfone membrane and polyvinylidene fluoride–titanium dioxide (PVDF–TiO2) mixed membranes, respectively. The former showed 99.3% dye (6 mg L−1) rejection at 300 kPa while the latter displayed 99% rejection at 0.5 bar. The mixed membrane was more economical because it can achieve high rejection at a low trans-membrane pressure. PVDF–TiO2 also possesses significant UV cleaning properties which simplify its handling.
| Membrane | Dyes | Removal (%) | Conditions | Reference (s) |
|---|---|---|---|---|
| NF 200, NF270 | Everzol Black, Everzol Blue, Everzol Red | >90 | Initial dye concentration: 600 mg L−1, pressure: 3–12 bar | 143 |
| PMIA | Eriochrome Black T | >99 | Initial dye concentration: 1 g L−1, pressure: 0.4 MPa, 1 g L−1 NaCl | 148 |
| Acrylic grafted polysulfone | 9 textile dyes | 86–99 | Initial dye concentration: 50 mg L−1 | 149 |
| CMCNa/PP thin film composite (700 Da) | Sunset Yellow | 82.2 | Initial dye concentration: 100 mg L−1, pH: 6.8, pressure: 0.8 bar, flux: 6.2–6.9 L m−2 h−1 | 150 |
| Methyl blue | 99.7 | |||
| Congo Red | 99.9 | |||
| Polysulfone–polyamide thin film | Reactive Black 5 | 60–97 | Initial dye concentration: 0.4–2 g L−1 pressure: 5–25 psi | 151 |
| CMCNa/PP thin film composite (700 Da) | Congo Red, methyl blue | 99.9 | Initial dye concentration: 100 mg L−1, pH: 6.8, pressure: 0.8 bar, flux: 6.2–6.9 L m−2 h−1 | 150 |
| UV grafting on sPPSU (1627–1674 Da) | Safranin O, Orange II | 99.98, 86.76 | Initial dye concentration: 50 mg L−1, 30 min, pressure: 5 bar | 152 |
Several novel nanofiltration membranes with exciting features have been developed by researchers. Two positively charged nanofiltration membranes were prepared by Zhong et al.152 through UV grafting on sulfonated polyphenylenesulfone (sPPSU) at different UV exposures. The membranes have better rejection with positively charged dyes compared to negatively charged dyes. The differential duration of UV exposure was found to affect the flux and rejection of the resulting membranes. Increasing duration of UV exposure reduced the permeate flux while bringing rejection to a maximum, then decreasing at longer UV exposure. Liu et al.153 developed new sulfonated thin-film composite nanofiltration membranes which enhanced the water permeability up to 38–54% without reducing rejection performance. Shao et al.154 also developed a novel nanofiltration composite membrane through interfacial polymerization. The membrane was able to remove more than 90% of Safranin O and Aniline Blue dyes at pH 11.
3.8. Biological treatment
Biological treatment procedures for the removal of contaminations from wastewater are considered highly useful due to their eco-friendly nature, minimum usage of chemicals and energy saving nature. The principle of the biological treatment procedures is the conversion of biodegradable wastes into simpler and harmless species through biological processes by various microorganisms. The treatment processes can be categorized into aerobic or anaerobic process. Usually both processes are conducted for wastewater treatment. The organisms used may be bacteria, fungi, algae or plants. Enzyme systems are also used in biological treatment. The final products after aerobic treatment are carbon dioxide, water and biomass, while the final products after anaerobic treatment are carbon dioxide, methane and biomass. This treatment is able to treat dye solutions in an environmental friendly way without high investment and cost.Bacteria not only decolorize dye solution but also mineralize and degrade many dyes, which is inexpensive and eco-friendly. Kumar Garg et al.155 studied the potential of Pseudomonas putida SKG-1 isolate for the removal of Orange II dye, and stressed the need to optimize culture and nutritional conditions. 92.8% dye (100 mg L−1) removal was achieved in 96 hours at pH 8, a temperature of 30 °C and static conditions. Jadhav et al.156 used Pseudomonas aeruginosa to decolorize 97% Remazol Red (50 mg L−1) in 20 min at pH 7, a temperature of 40 °C and static conditions. The method was able to treat solutions with dye concentration up to 250 mg L−1. However, repeated cycles were found to cause significant drops in decoloration, and increased time, which might be due to the depletion of essential nutrients for bacterial activities.
Using a single bacterial isolate to remove dyes may not give satisfactory results. Paul et al.157 investigated the effect of applying irradiation before Pseudomonas sp. SUK1 degradation of Reactive Red 120. At lower doses of irradiation (≤1 kGy), decoloration and mineralization were improved significantly. 98% decoloration and 90% TOC removal were achieved using 1 kGy irradiation after 96 hours microbial treatment.
Besides applying other physical or chemical processes, the use of a bacterial consortium can also improve dye removal performance. Dye removal potential of a bacterial consortium EDPA consisting of Enterobacter dissolvens AGYP1 and Pseudomonas aeruginosa AGYP2 was tested by Patel et al.158 and 93% Acid Red 119 (100 mg L−1) removal after 20 hours at pH 7 at static conditions was reported. This bacterial consortium was able to decolorize high concentrations of Acid Maroon V (up to 2000 mg L−1) and also decolorized 16 other dyes. A bacterial consortium with rice husks as a support and carbon source was studied by Forss et al.159 to remove Reactive Red 2 and Reactive Black 5. About 80% decoloration was achieved in 28.4 hours using this newly designed bio-filter system. Phugare et al.160 used a bacterial consortium SDS containing Providencia sp. and Pseudomonas aeruginosa to remove Reactive Red 120. Complete removal was achieved using 50 mg L−1 dye concentration in 1 hour at pH 7 and static conditions. Good detoxification properties were also reported. On the other hand, Senthilkumar et al.129 reported that a bacterial consortium with bacteria isolated from textile site soil only gave 42.2% Proncion Scarlet dye removal. The combination of chemical oxidation with bacterial degradation significantly improved decoloration to more than 96% with high COD removal.
Although some researchers claim that fungi are not as effective as bacteria for dye removal, there are several reports indicating high dye removal efficiencies using fungi. Kumar et al.161 used Aspergillus sp. to remove Brilliant Green dye. 99.2% dye (10 mg L−1) removal was reached within 72 hour at pH 5, a temperature of 35 °C and with agitation. This method was said to be low cost and have simple handling. Several white root fungi were studied by Kalpana et al.162 to remove Reactive Levafix Blue E-RA. Irpex lacteus was identified as the best one and was able to give complete decoloration without producing toxic metabolites. Lade et al.163 tested a fungal-bacterial consortium consisting of Aspergillus ochraceus and Pseudomonas sp. to remove Rubine GFL dye. This consortium enables both fungal and bacterial strains to complement each other and enhances overall performance. 95% dye (100 mg L−1) removal was achieved in 30 hours at pH 8.5, a temperature of 37 °C and under micro-aerophilic conditions. Khataee and Dehghan164 used microalgae Chara sp. and Cladophora sp., respectively, for removal of malachite green. Neural network analysis was conducted to predict dye degradation using Chara sp. while RSM optimization was used to optimize Cladophora sp. dye removal performance. Results showed more than 90% dye removal using Chara sp. and 71% dye removal using Cladophora sp.
Knowledge of the enzymes responsible for dye degradation may be very helpful in designing biological wastewater treatment technologies. Enzyme based treatments can reduce biological contamination caused by microbes used during biological treatment. Yang et al.165 studied an integrated enzyme system to treat methyl red solution. Up to 85% removal was achieved by optimizing four parameters viz. enzyme ratio, dye concentration, NAD+ and glucose concentration. Vafaei et al.166 used an aquatic fern, Azolla filiculoides, to remove Basic Red 46. Effects of duration, dye concentration, fresh fern weight, pH and temperature were investigated. A best removal of 99% was achieved within 7 days.
Bioreactor treatments could give better performance compared to conventional activated sludge treatments. Several bioreactors or equipment are being studied such as a sequencing batch bio-filter granular reactor (SBBGR),167 sequencing batch reactor (SBR), sequencing batch bio-film reactor,168 moving bed sequencing batch bio-film reactor (MB-SBBR)169 and membrane aerated biofilm reactor (MABR).170 Anaerobic and aerobic conditions were investigated by the researchers to determine the best conditions for dye removal. For instance, Hosseini et al.168 showed the high efficiency of anaerobic SBR for removing Acid Red 18 for the long term. After anaerobic SBR, aerobic MB-SBBR was used to remove metabolites produced during anaerobic degradation of the dye.169 MABR also showed high Acid Orange 7 decoloration (98%) and COD removal in 6 hours at optimized conditions.170 In order to enhance the biological treatment of textile effluents containing dyes, Lotito et al.167 integrated the process with ozonation and more than 80% removal was achieved. Micro-electrolysis was used by Huang et al.171 to enhance anaerobic treatment of Reactive Blue 19 with 65% removal. Applying an external electric field further improved the removal up to 90%. These studies showed that various approaches can be applied to enhance the biological treatments of dyes.
3.9. Combined techniques
Since each dye removal technique has its own advantages and disadvantages, these techniques can be combined together to complement each other for better dye removal efficiency. Basha et al.172 combined electrochemical oxidation, microbial oxidation, electrolysis and photocatalytic oxidation sequentially to remove Proncion Blue, which takes 5 days and 14 hours to give high removal. 92–95% and 80–93% removals were achieved using bacterial strains and fungal strains, respectively, during microbial oxidation.Membranes are very useful in preventing wash out. Therefore, they are widely used to improve other dye removal techniques. Some studies involved the use of a photocatalysis/UF hybrid to recover a TiO2 catalyst during operation,173 whole cell fungal membrane bio-reactors where the membrane reduces enzyme wash out and enhances solid–liquid separation,174 and an aerobic membrane bio-reactor where the membrane helps to retain dye molecules for biological treatment.175
In some cases, other techniques can be used to enhance membrane filtration. Xu et al.176 applied electrolytic oxidation to nanofiltration to enable lower operating pressure and membrane area. On the other hand, Vergili et al.177 studied the combination of various filtration techniques (ultrafiltration, loose nanofiltration, tight nanofiltration, and reverse osmosis) on dye removal. Comparative economic analysis was also done and the choice depends on the desired effluent quality and budget.
The studies mentioned above show that various improvements such as longer retention time, better decoloration, better detoxification, recovery of valuable compounds, and lower costs can be obtained by combining techniques. Therefore, combination strategies are a novel and important window of research for the development of efficient technologies for the removal of dyes from wastewater.
4. Future perspectives
A critical analysis of the discussion in this review indicates significantly increasing advancements in dye removal techniques. In response to increasingly stringent environmental regulations and public awareness, new studies on dye removal techniques need to show good performance complying within the regulatory limits imposed. The three most frequent dye removal techniques include chemical oxidation, adsorption and biological treatment. Chemical oxidation is able to give excellent decoloration within a short duration. When combined with other techniques, high mineralization can be achieved. Adsorption provides high flexibility for dye removal. A wide range of adsorbents including magnetic nanoparticles and low cost adsorbents have been studied. However, out of the large number of research articles being continuously published on adsorption technology, a few points must be considered for future research, such as pilot plant adsorption studies, and handling of the adsorbent, especially nanosorbents. Separation and aggregation of nanomaterials is also a hurdle for use in actual systems. Release of the nanoadsorbent in aqueous solution causes nanotoxicity to living systems. Regeneration of spent adsorbent is still a big issue which needs to be focused on.Biological treatment is an easy and environmental friendly way to treat dye wastewater. Various advances including bioreactor studies are being carried out to improve its performance. Nevertheless, selection of the most suitable technique would depend on the effluent conditions, type of dye, operating conditions, treatment quality needed, costs, flexibility, environmental impact, and others. Biological treatment is a time consuming and uncontrolled process. More research work should be focused on reduction in the degradation period under a controlled manner. Biotreatments are versatile but should be modified according to the target pollutant.
Chemical precipitation can be used to treat dye solutions with high removal efficiency. Dye concentrations ranging from 25–300 mg L−1 can be treated. A main obstacle is that the chemical price may become a great concern for using this technique as high chemical doses are needed for the treatment of high volumes of industrial wastewater. Besides, the production of a large amount of sludge is also a big concern. Coagulation–flocculation is one of the conventional methods for dye removal. Dye concentrations ranging from 60–400 mg L−1 and 800–1500 mg L−1 can be removed. This technique enables efficient dye removal and is relatively simple to operate. However, chemical cost and sludge production are the two factors of concern for this dye removal technique. Besides, dye molecules tend to form complexes with flocculants which further hinders the potential of dye recovery afterwards. Chemical oxidation techniques such as ozonation, photocatalytic oxidation, and the Fenton process enable the removal of dyes ranging from 50–400 mg L−1 and up to 1000 mg L−1. This method gives an excellent decoloration within a short time. However, degradation of dyes may give compounds which are more toxic than those before treatment. Therefore, combination of other treatment techniques is suggested to enhance the mineralization of dyes. Photocatalytic degradation/photodegradation needs to mainly focus on the utilization of visible light. In future, visible light active photocatalysts must be developed for the degradation of organic pollutants like dyes.
Electrochemical treatment techniques are effective in both decoloration and mineralization. Dye concentrations ranging from 50–200 mg L−1 and 400–2000 mg L−1 can be removed using this technology. The energy consumption is a major limitation of this treatment technology. Membrane filtration can effectively remove high percentages of dyes while recovering the water for re-use. Dye concentrations ranging from 6–5000 mg L−1 can be treated by using membrane filtration. The main limitation with this treatment technique is that the process is very complex and membrane fouling is another big problem. Regular cleaning and concentrated sludge production are also other issues related to membrane filtration. Membrane filtration is a most promising technology for the treatment of wastewaters. Currently a lot of researchers are working on the development of low cost membranes with high mechanical strength.
5. Conclusions
Dyes are an important class of pollutants and affect general human and aquatic life drastically. In order to reduce the negative impacts of dye contaminated water on humans and the environment, the wastewater must be treated carefully before discharge. Several dye removal strategies including conventional techniques such as adsorption, oxidation, flocculation–coagulation and biological treatment, as well as relatively new techniques such as reverse micellar extraction have been developed for the removal of dyes from wastewater. It may be understood from the discussion in this review that chemical oxidation, adsorption and biological treatment are the most frequently investigated techniques over the past few years. Indeed, the newer techniques are bringing about several improvements. Most of the techniques are able to achieve more than 80% dye removal and several exceed 90%. Really, great advances in the removal of dyes from wastewater have been reported during the last few years and it is quite encouraging that several reported methods are very fast, have low costs with exciting dye removal efficiencies. Therefore, it is advised that more research be carried out in this direction because water is the second most precious abiotic component of our ecosystem and its safe treatment and conservation is the duty of every person living on this planet. We hope that more sophisticated technologies are developed so that wastewater can be treated easily, with low costs, at both industrial and pilot scales.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors acknowledge the Research Management Centre (RMC) and financial support from ZAMALAH of Universiti Teknologi Malaysia.References
- P. F. Gordon and P. Gregory, Organic Chemistry in Colour, Springer-Verlag, Berlin Heidelberg, New York, 1983 Search PubMed.
- G. Moussavi and M. Mahmoudi, J. Hazard. Mater., 2009, 168, 806–812 CrossRef CAS PubMed.
- I. Bazin, A. Ibn Hadj Hassine, Y. Haj Hamouda, W. Mnif, A. Bartegi, M. Lopez-Ferber, M. De Waard and C. Gonzalez, Ecotoxicol. Environ. Saf., 2012, 85, 131–136 CrossRef CAS PubMed.
- I. Holme, Ecological aspects of color chemistry, Developments in the Chemistry and Technology of Organic Dyes, Society of Chemistry Industry, Oxford, 1st edn, 1984, pp. 1–128 Search PubMed.
- N. O. San, A. Celebioglu, Y. Tumtas, T. Uyar and T. Tekinay, RSC Adv., 2014, 4, 32249 RSC.
- T. Robinson, G. McMullan, R. Marchant and P. Nigam, Bioresour. Technol., 2001, 77, 247–255 CrossRef CAS.
- C. Moran, M. E. Hall and R. C. Howell, J. Soc. Dyers Colour., 1997, 113, 272–274 CrossRef CAS PubMed.
- N. Nemerow and A. Dasgupta, Industrial and Hazardous Waste Treatment, Van Nostrand Reinhold, New York, 1991 Search PubMed.
- G. Tchobanoglous and L. B. Franklin, Wastewater Engineering: Treatment, Disposal and Reuse, McGraw Hill, Inc., New York, 1991 Search PubMed.
- I. Ali and H. Y. Aboul-Enein, Chiral Pollutants: Distribution, Toxicity and Analysis by Chromatography and Capillary Electrophoresis, John Wiley & Sons, Chichester, UK, 2004 Search PubMed.
- V. K. Gupta and Suhas, J. Environ. Manage., 2009, 90, 2313–2342 CrossRef CAS PubMed.
- A. Ahmad, M. Rafatullah, O. Sulaiman, M. H. Ibrahim and R. Hashim, J. Hazard. Mater., 2009, 170, 357–365 CrossRef CAS PubMed.
- T. Ahmad, M. Rafatullah, A. Ghazali, O. Sulaiman and R. Hashim, J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev., 2011, 29, 177–222 CrossRef CAS PubMed.
- T. Ahmad, M. Danish, M. Rafatullah, A. Ghazali, O. Sulaiman, R. Hashim and M. N. Ibrahim, Environ. Sci. Pollut. Res. Int., 2012, 19, 1464–1484 CrossRef CAS PubMed.
- A. Demirbas, J. Hazard. Mater., 2009, 167, 1–9 CrossRef CAS PubMed.
- I. Ali, Chem. Rev., 2012, 112, 5073–5091 CrossRef CAS PubMed.
- S. Batool, S. Akib, M. Ahmad, K. S. Balkhair and M. A. Ashraf, J. Nanomater., 2014, 20 Search PubMed , ID 864914.
- A. K. Verma, R. R. Dash and P. Bhunia, J. Environ. Manage., 2012, 93, 154–168 CrossRef CAS PubMed.
- L. Charalampos, D. Danae and G. Konstantinos, Sep. Purif. Rev., 2015, 44, 74–107 CrossRef.
- V. V. Panic, S. I. Seslija, A. R. Nesic and S. J. Velickovic, Hem. Ind., 2013, 67, 881–900 CrossRef CAS.
- V. Kuokkanen, T. Kuokkanen, J. Ramo and U. Lassi, Green Sustainable Chem., 2013, 3, 89–121 CrossRef.
- S. Palit, Int. J. Chem. Sci., 2012, 10, 27–35 CAS.
- A. K. Jain, V. K. Gupta, A. Bhatnagar and Suhas, J. Hazard. Mater., 2003, B101, 31–42 CrossRef.
- M. Rafatullah, O. Sulaiman, R. Hashim and A. Ahmad, J. Hazard. Mater., 2010, 177, 70–80 CrossRef CAS PubMed.
- R. Kumar, J. Rashid and M. A. Barakat, RSC Adv., 2014, 4, 38334 RSC.
- G. Z. Kyzas, N. K. Lazaridis and A. C. Mitropoulos, Chem. Eng. J., 2012, 189–190, 148–159 CrossRef CAS PubMed.
- J. M. Dias, M. C. M. Alvim-Ferraz, M. F. Almeida, J. Rivera-Utrilla and M. Sánchez-Polo, J. Environ. Manage., 2007, 85, 833–846 CrossRef CAS PubMed.
- M. E. Fernandez, G. V. Nunell, P. R. Bonelli and A. L. Cukierman, Ind. Crops Prod., 2014, 62, 437–445 CrossRef CAS PubMed.
- G. Mezohegyi, F. P. van der Zee, J. Font, A. Fortuny and A. Fabregat, J. Environ. Manage., 2012, 102, 148–164 CrossRef CAS PubMed.
- V. O. Njoku, K. Y. Foo, M. Asif and B. H. Hameed, Chem. Eng. J., 2014, 250, 198–204 CrossRef CAS PubMed.
- Z. Emami and S. Azizian, J. Anal. Appl. Pyrolysis, 2014, 108, 176–184 CrossRef CAS PubMed.
- H. Trevino-Cordero, L. G. Juarez-Aguilar, D. I. Mendoza-Castillo, V. Hernandez-Montoya, A. Bonilla-Petriciolet and M. A. Montes-Moran, Ind. Crops Prod., 2013, 42, 315–323 CrossRef CAS PubMed.
- V. K. Gupta, B. Gupta, A. Rastogi, S. Agarwal and A. Nayak, J. Hazard. Mater., 2011, 186, 891–901 CrossRef CAS PubMed.
- P. Hadi, M. Xu, C. Ning, C. S. K. Lin and G. McKay, Chem. Eng. J., 2015, 260, 895–906 CrossRef CAS PubMed.
- W. Li, Q. Yue, P. Tu, Z. Ma, B. Gao, J. Li and X. Xu, Chem. Eng. J., 2011, 178, 197–203 CrossRef CAS PubMed.
- L. P. Kong, X. J. Gan, A. L. B. Ahmad, B. H. Hamed, E. R. Evarts, B. S. Ooi and J. K. Lim, Chem. Eng. J., 2012, 197, 350–358 CrossRef CAS PubMed.
- H. Xu, Y. Zhang, Q. Jiang, N. Reddy and Y. Yang, J. Environ. Manage., 2013, 125, 33–40 CrossRef CAS PubMed.
- S. K. Giri, N. N. Das and G. C. Pradhan, Colloids Surf., A, 2011, 389, 43–49 CrossRef CAS PubMed.
- A. Debrassi, A. F. Correa, T. Baccarin, N. Nedelko, A. Slawska-Waniewska, K. Sobczak, P. Dłużewski, J. M. Greneche and C. A. Rodrigues, Chem. Eng. J., 2012, 183, 284–293 CrossRef CAS PubMed.
- K. A. Tan, N. Morad, T. T. Teng, I. Norli and P. Panneerselvam, APCBEE Proc., 2012, 1, 83–89 CrossRef CAS PubMed.
- S. Shariati, M. Faraji, Y. Yamini and A. A. Rajabi, Desalination, 2011, 270, 160–165 CrossRef CAS PubMed.
- L. Fan, C. Luo, M. Sun, H. Qiu and X. Li, Colloids Surf., B, 2013, 103, 601–607 CrossRef CAS PubMed.
- L. Lian, X. Cao, Y. Wu, D. Lou and D. Han, J. Taiwan Inst. Chem. Eng., 2013, 44, 67–73 CrossRef CAS PubMed.
- N. M. Mahmoodi, J. Taiwan Inst. Chem. Eng., 2013, 44, 322–330 CrossRef CAS PubMed.
- P. Panneerselvam, N. Morad, K. A. Tan and R. Mathiyarasi, Sep. Sci. Technol., 2012, 47, 742–752 CrossRef CAS.
- N. M. Mahmoodi, J. Ind. Eng. Chem., 2014, 20, 2050–2058 CrossRef CAS PubMed.
- S. M. Ahmed, F. I. El-Dib, N. S. El-Gendy, W. Mayed and M. El-Khodary, Arabian J. Chem., 2012 DOI:10.1016/j.arabjc.2012.04.049.
- Y. C. Lee, E. J. Kim, J. W. Yang and H. J. Shin, J. Hazard. Mater., 2011, 192, 62–70 CAS.
- P. Assefi, M. Ghaedi, A. Ansari, M. H. Habibi and M. S. Momeni, J. Ind. Eng. Chem., 2014, 20, 2905–2913 CrossRef CAS PubMed.
- Z. M. Magriotis, M. Z. Carvalho, P. F. de Sales, F. C. Alves, R. F. Resende and A. A. Saczk, J. Environ. Chem. Eng., 2014, 2, 1731–1740 CrossRef CAS PubMed.
- M. Ahmaruzzaman and V. K. Gupta, Ind. Eng. Chem. Res., 2011, 50, 13589–13613 CrossRef CAS.
- M. A. M. Salleh, D. K. Mahmoud, W. A. W. A. Karim and A. Idris, Desalination, 2011, 280, 1–13 CrossRef CAS PubMed.
- N. M. Mahmoodi, R. Salehi, M. Arami and H. Bahrami, Desalination, 2011, 267, 64–72 CrossRef CAS PubMed.
- Y. Djilali, E. H. Elandaloussi, A. Aziz and L. C. de Ménorval, J. Saudi Chem. Soc., 2012 DOI:10.1016/j.jscs.2012.10.013.
- Z. Zhang, I. M. O’Hara, G. A. Kent and W. O. S. Doherty, Ind. Crops Prod., 2013, 42, 41–49 CrossRef CAS PubMed.
- M. S. Sajab, C. H. Chia, S. Zakaria and P. S. Khiew, Bioresour. Technol., 2013, 128, 571–577 CrossRef CAS PubMed.
- T. Madrakian, A. Afkhami and M. Ahmadi, Spectrochim. Acta, Part A, 2012, 99, 102–109 CrossRef CAS PubMed.
- N. Barka, K. Ouzaouit, M. Abdennouri and M. E. Makhfouk, J. Taiwan Inst. Chem. Eng., 2013, 44, 52–60 CrossRef CAS PubMed.
- M. Asgher and H. N. Bhatti, Ecol. Eng., 2012, 38, 79–85 CrossRef PubMed.
- W. Zhang, H. Li, X. Kan, L. Dong, H. Yan, Z. Jiang, H. Yang, A. Li and R. Cheng, Bioresour. Technol., 2012, 117, 40–47 CrossRef CAS PubMed.
- M. Kousha, E. Daneshvar, A. R. Esmaeli, M. Jokar and A. R. Khataee, Int. Biodeterior. Biodegrad., 2012, 69, 97–105 CrossRef CAS PubMed.
- R. Kumar and R. Ahmad, Desalination, 2011, 265, 112–118 CrossRef CAS PubMed.
- R. Kumar and M. A. Barakat, Chem. Eng. J., 2013, 226, 377–383 CrossRef CAS PubMed.
- L. W. Low, T. T. Teng, M. Rafatullah, B. Azahari and N. Morad, Sep. Sci. Technol., 2013, 48, 1688–1698 CrossRef CAS.
- C. Kannan, K. Muthuraja and M. R. Devi, J. Hazard. Mater., 2013, 244–245, 10–20 CrossRef CAS PubMed.
- M. Kousha, E. Daneshvar, M. S. Sohrabi, M. Jokar and A. Bhatnagar, Chem. Eng. J., 2012, 192, 67–76 CrossRef CAS PubMed.
- M. Greluk and Z. Hubicki, Desalination, 2011, 278, 219–226 CrossRef CAS PubMed.
- Y. Wen, C. Shen, Y. Ni, S. Tong and F. Yu, J. Hazard. Mater., 2012, 201–202, 162–169 CrossRef CAS PubMed.
- C. Meziti and A. Boukerroui, Procedia Eng., 2012, 33, 303–312 CrossRef CAS PubMed.
- A. Islam, M. A. Laskar and A. Ahmad, Talanta, 2010, 81, 1772–1780 CrossRef CAS PubMed.
- N. M. Mahmoodi, F. Najafi and A. Neshat, Ind. Crops Prod., 2013, 42, 119–125 CrossRef CAS PubMed.
- V. V. Panic, Z. P. Madzarevic, T. Volkov-Husovic and S. J. Velickovic, Chem. Eng. J., 2013, 217, 192–204 CrossRef CAS PubMed.
- E. A. Abdullah, A. H. Abdullah, Z. Zainal, M. Z. Hussein and T. K. Ban, J. Environ. Sci., 2012, 24, 1876–1884 CrossRef CAS.
- N. Mohammadi, H. Khani, V. K. Gupta, E. Amereh and S. Agarwal, J. Colloid Interface Sci., 2011, 362, 457–462 CrossRef CAS PubMed.
- M. Greluk and Z. Hubicki, Chem. Eng. Res. Des., 2013, 91, 1343–1351 CrossRef CAS PubMed.
- M. Greluk and Z. Hubicki, Chem. Eng. J., 2013, 215–216, 731–739 CrossRef CAS PubMed.
- M. Wawrzkiewicz, Chem. Eng. J., 2011, 173, 773–781 CrossRef CAS PubMed.
- M. Wawrzkiewicz, Chem. Eng. J., 2013, 217, 414–425 CrossRef CAS PubMed.
- C. Shuang, P. Li, A. Li, Q. Zhou, M. Zhang and Y. Zhou, Water Res., 2012, 46, 4417–4426 CrossRef CAS PubMed.
- M. Constantin, I. Asmarandei, V. Harabagiu, L. Ghimici, P. Ascenzi and G. Fundueanu, Carbohydr. Polym., 2013, 91, 74–84 CrossRef CAS PubMed.
- E. Alver and A. U. Metin, Chem. Eng. J., 2012, 200–202, 59–67 CrossRef CAS PubMed.
- A. Z. Bouyakoub, B. S. Lartiges, R. Ouhib, S. Kacha, A. G. El Samrani, J. Ghanbaja and O. Barres, J. Hazard. Mater., 2011, 187, 264–273 CrossRef CAS PubMed.
- G. Zhang, X. Li, Y. Li, T. Wu, D. Sun and F. Lu, Desalination, 2011, 274, 255–261 CrossRef CAS PubMed.
- N. A. Oladoja, I. O. Raji, S. E. Olaseni and T. D. Onimisi, Chem. Eng. J., 2011, 171, 941–950 CrossRef CAS PubMed.
- K. E. Lee, N. Morad, T. T. Teng and B. T. Poh, APCBEE Proc., 2012, 1, 59–65 CrossRef CAS PubMed.
- I. Khouni, B. Marrot, P. Moulin and R. B. Amar, Desalination, 2011, 268, 27–37 CrossRef CAS PubMed.
- M. Khayet, A. Y. Zahrim and N. Hilal, Chem. Eng. J., 2011, 167, 77–83 CrossRef CAS PubMed.
- S. S. Moghaddam, M. R. Moghaddam and M. Arami, J. Environ. Manage., 2011, 92, 1284–1291 CrossRef PubMed.
- H. Patel and R. T. Vashi, J. Saudi Chem. Soc., 2012, 16, 131–136 CrossRef CAS PubMed.
- B. Merzouk, B. Gourich, K. Madani, C. Vial and A. Sekki, Desalination, 2011, 272, 246–253 CrossRef CAS PubMed.
- G. M. Ayoub, A. Hamzeh and L. Semerjian, Desalination, 2011, 273, 359–365 CrossRef CAS PubMed.
- L. F. Albuquerque, A. A. Salgueiro, J. L. d. S. Melo and O. Chiavone-Filho, Sep. Purif. Technol., 2013, 104, 246–249 CrossRef CAS PubMed.
- C. Wu, Y. Wang, B. Gao, Y. Zhao and Q. Yue, Sep. Purif. Technol., 2012, 95, 180–187 CrossRef CAS PubMed.
- Y. F. Wang, B. Y. Gao, Q. Y. Yue, Y. Wang and Z. L. Yang, Bioresour. Technol., 2012, 113, 265–271 CrossRef CAS PubMed.
- V. Mezzanotte, R. Fornaroli, S. Canobbio, L. Zoia and M. Orlandi, Chemosphere, 2013, 91, 629–634 CrossRef CAS PubMed.
- N. M. Mahmoodi, Desalination, 2011, 279, 332–337 CrossRef CAS PubMed.
- M. L. Marin, L. Santos-Juanes, A. Arques, A. M. Amat and M. A. Miranda, Chem. Rev., 2012, 112, 1710–1750 CrossRef CAS PubMed.
- R. K. Upadhyay, N. Soin and S. S. Roy, RSC Adv., 2014, 4, 3823–3851 RSC.
- V. K. Gupta, R. Jain, S. Agarwal and M. Shrivastava, Colloids Surf., A, 2011, 378, 22–26 CrossRef CAS PubMed.
- V. K. Gupta, R. Jain, A. Nayak, S. Agarwal and M. Shrivastava, Mater. Sci. Eng., C, 2011, 31, 1062–1067 CrossRef CAS PubMed.
- V. K. Gupta, R. Jain, S. Agarwal, A. Nayak and M. Shrivastava, J. Colloid Interface Sci., 2012, 366, 135–140 CrossRef CAS PubMed.
- L. Karimi, S. Zohoori and M. E. Yazdanshenas, J. Saudi Chem. Soc., 2014, 18, 581–588 CrossRef CAS PubMed.
- M. L. Yola, T. Eren, N. Atar and S. Wang, Chem. Eng. J., 2014, 242, 333–340 CrossRef CAS PubMed.
- R. Yuan, S. N. Ramjaun, Z. Wang and J. Liu, Chem. Eng. J., 2012, 192, 171–178 CrossRef CAS PubMed.
- Q. Zhang, Y. H. Jing, A. Shiue, C. T. Chang, B. Y. Chen and C. C. Hsueh, J. Taiwan Inst. Chem. Eng., 2012, 43, 760–766 CrossRef CAS PubMed.
- T. A. Saleh and V. K. Gupta, J. Colloid Interface Sci., 2012, 371, 101–106 CrossRef CAS PubMed.
- T. Yan, L. Li, G. Li, Y. Wang, W. Hu and X. Guan, J. Hazard. Mater., 2011, 186, 272–279 CrossRef CAS PubMed.
- R. K. Upadhyay, M. Sharma, D. K. Singh, S. S. Amritphale and N. Chandra, Sep. Purif. Technol., 2012, 88, 39–45 CrossRef CAS PubMed.
- P. Li, G. Zhao, K. Zhao, J. Gao and T. Wu, Dyes Pigm., 2012, 92, 923–928 CrossRef CAS PubMed.
- G. Zhang, S. Wang, S. Zhao, L. Fu, G. Chen and F. Yang, Appl. Catal., B, 2011, 106, 370–378 CrossRef CAS PubMed.
- C. Shen, Y. Wen, Z. Shen, J. Wu and W. Liu, Facile, J. Hazard. Mater., 2011, 193, 209–215 CrossRef CAS PubMed.
- Y. Dong, W. Dong, Y. Cao, Z. Han and Z. Ding, Catal. Today, 2011, 175, 346–355 CrossRef CAS PubMed.
- R. K. Sharma, S. Gulati, A. Pandey and A. Adholeya, Appl. Catal., B, 2012, 125, 247–258 CrossRef CAS PubMed.
- T. S. Natarajan, M. Thomas, K. Natarajan, H. C. Bajaj and R. J. Tayade, Chem. Eng. J., 2011, 169, 126–134 CrossRef CAS PubMed.
- J. Zhang, M. Feng, Y. Jiang, M. Hu, S. Li and Q. Zhai, Chem. Eng. J., 2012, 191, 236–242 CrossRef CAS PubMed.
- L. Liu, J. Zhang, Y. Tan, Y. Jiang, M. Hu, S. Li and Q. Zhai, Chem. Eng. J., 2014, 244, 9–18 CrossRef CAS PubMed.
- V. Dulman, S.M. Cucu-Man, R. I. Olariu, R. Buhaceanu, M. Dumitraş and I. Bunia, Dyes Pigm., 2012, 95, 79–88 CrossRef CAS PubMed.
- J. R. Guimaraes, M. G. Maniero and R. N. de Araujo, J. Environ. Manage., 2012, 110, 33–39 CrossRef CAS PubMed.
- S. Yang, P. Wang, X. Yang, L. Shan, W. Zhang, X. Shao and R. Niu, J. Hazard. Mater., 2010, 179, 552–558 CrossRef CAS PubMed.
- R. Yuan, S. N. Ramjaun, Z. Wang and J. Liu, J. Hazard. Mater., 2011, 196, 173–179 CrossRef CAS PubMed.
- A. Safavi and S. Momeni, J. Hazard. Mater., 2012, 201–202, 125–131 CrossRef CAS PubMed.
- L. O. Benetoli, B. M. Cadorin, V. Z. Baldissarelli, R. Geremias, I. G. de Souza and N. A. Debacher, J. Hazard. Mater., 2012, 237–238, 55–62 CrossRef CAS PubMed.
- Z. Eren, J. Environ. Manage., 2012, 104, 127–141 CrossRef CAS PubMed.
- M. Geng and S. M. Thagard, Ultrason. Sonochem., 2013, 20, 618–625 CrossRef CAS PubMed.
- L. Hua, H. Ma and L. Zhang, Chemosphere, 2013, 90, 143–149 CrossRef CAS PubMed.
- G. Ovejero, A. Rodríguez, A. Vallet and J. Garcia, Chem. Eng. J., 2013, 215–216, 168–173 CrossRef CAS PubMed.
- A. Vallet, G. Ovejero, A. Rodriguez, J. A. Peres and J. Garcia, J. Hazard. Mater., 2013, 244–245, 46–53 CrossRef CAS PubMed.
- A. R. Amani-Ghadim, S. Aber, A. Olad and H. Ashassi-Sorkhabi, Chem. Eng. Process., 2013, 64, 68–78 CrossRef CAS PubMed.
- S. Senthilkumar, C. A. Basha, M. Perumalsamy and H. J. Prabhu, Electrochim. Acta, 2012, 77, 171–178 CrossRef CAS PubMed.
- S. Prakash, A. M. Rajesh and V. K. Shahi, Chem. Eng. J., 2011, 168, 108–114 CrossRef CAS PubMed.
- B. K. Korbahti, K. Artut, C. Geçgel and A. Ozer, Chem. Eng. J., 2011, 173, 677–688 CrossRef PubMed.
- E. Rosales, M. Pazos and M. Á. Sanroman, Desalination, 2011, 278, 312–317 CrossRef CAS PubMed.
- B. Vahid and A. Khataee, Electrochim. Acta, 2013, 88, 614–620 CrossRef CAS PubMed.
- H. An, H. Cui, W. Zhang, J. Zhai, Y. Qian, X. Xie and Q. Li, Chem. Eng. J., 2012, 209, 86–93 CrossRef CAS PubMed.
- M. Rivera, M. Pazos and M. Á. Sanroman, Desalination, 2011, 274, 39–43 CrossRef CAS PubMed.
- C. Ramirez, A. Saldana, B. Hernández, R. Acero, R. Guerra, S. Garcia-Segura, E. Brillas and J. M. Peralta-Hernandez, J. Ind. Eng. Chem., 2013, 19, 571–579 CrossRef CAS PubMed.
- P. Kariyajjanavar, N. Jogttappa and Y. A. Nayaka, J. Hazard. Mater., 2011, 190, 952–961 CrossRef CAS PubMed.
- M. Siddique, R. Farooq, Z. M. Khan, Z. Khan and S. F. Shaukat, Ultrason. Sonochem., 2011, 18, 190–196 CrossRef CAS PubMed.
- M. Riera-Torres and C. Gutiérrez-Bouzan, Sep. Purif. Technol., 2012, 98, 375–382 CrossRef CAS PubMed.
- A. Somayajula, P. Asaithambi, M. Susree and M. Matheswaran, Ultrason. Sonochem., 2012, 19, 803–811 CrossRef CAS PubMed.
- A. I. del Río, J. Fernández, J. Molina, J. Bonastre and F. Cases, Desalination, 2011, 273, 428–435 CrossRef PubMed.
- Y. Zheng, G. Yao, Q. Cheng, S. Yu, M. Liu and C. Gao, Desalination, 2013, 328, 42–50 CrossRef CAS PubMed.
- A. Aouni, C. Fersi, B. Cuartas-Uribe, A. Bes-Pía, M. I. Alcaina-Miranda and M. Dhahbi, Desalination, 2012, 297, 87–96 CrossRef CAS PubMed.
- E. Alventosa-de Lara, S. Barredo-Damas, M. I. Alcaina-Miranda and M. I. Iborra-Clar, J. Hazard. Mater., 2012, 209–210, 492–500 CrossRef CAS PubMed.
- Y. Dong, Y. Su, W. Chen, J. Peng, Y. Zhang and Z. Jiang, Chin. J. Chem. Eng., 2011, 19, 863–869 CrossRef CAS.
- S. Mondal, H. Ouni, M. Dhahbi and S. De, J. Hazard. Mater., 2012, 229–230, 381–389 CrossRef CAS PubMed.
- H. P. Ngang, B. S. Ooi, A. L. Ahmad and S. O. Lai, Chem. Eng. J., 2012, 197, 359–367 CrossRef CAS PubMed.
- J. Huang and K. Zhang, Desalination, 2011, 282, 19–26 CrossRef CAS PubMed.
- M. Amini, M. Arami, N. M. Mahmoodi and A. Akbari, Desalination, 2011, 267, 107–113 CrossRef CAS PubMed.
- S. Yu, Z. Chen, Q. Cheng, Z. Lu, M. Liu and C. Gao, Sep. Purif. Technol., 2012, 88, 121–129 CrossRef CAS PubMed.
- S. K. Maurya, K. Parashuram, P. S. Singh, P. Ray and A. V. R. Reddy, Desalination, 2012, 304, 11–19 CrossRef CAS PubMed.
- P. S. Zhong, N. Widjojo, T. S. Chung, M. Weber and C. Maletzko, J. Membr. Sci., 2012, 417–418, 52–60 CrossRef CAS PubMed.
- Y. Liu, S. Zhang, Z. Zhou, J. Ren, Z. Geng, J. Luan and G. Wang, J. Membr. Sci., 2012, 394–395, 218–229 CrossRef CAS PubMed.
- L. Shao, X. Q. Cheng, Y. Liu, S. Quan, J. Ma, S. Z. Zhao and K. Y. Wang, J. Membr. Sci., 2013, 430, 96–105 CrossRef CAS PubMed.
- G. Kumar, M. Tripathi, S. K. Singh and J. K. Tiwari, Int. Biodeterior. Biodegrad., 2012, 74, 24–35 CrossRef PubMed.
- S. B. Jadhav, S. S. Phugare, P. S. Patil and J. P. Jadhav, Int. Biodeterior. Biodegrad., 2011, 65, 733–743 CrossRef CAS PubMed.
- J. Paul, A. A. Kadam, S. P. Govindwar, P. Kumar and L. Varshney, Chemosphere, 2013, 90, 1348–1358 CrossRef CAS PubMed.
- Y. Patel, C. Mehta and A. Gupte, Int. Biodeterior. Biodegrad., 2012, 75, 187–193 CrossRef CAS PubMed.
- J. Forss, J. Pinhassi, M. Lindh and U. Welander, Bioresour. Technol., 2013, 130, 681–688 CrossRef CAS PubMed.
- S. S. Phugare, D. C. Kalyani, A. V. Patil and J. P. Jadhav, J. Hazard. Mater., 2011, 186, 713–723 CrossRef CAS PubMed.
- C. G. Kumar, P. Mongolla, J. Joseph and V. U. M. Sarma, Process Biochem., 2012, 47, 1388–1394 CrossRef CAS PubMed.
- D. Kalpana, N. Velmurugan, J. H. Shim, B. T. Oh, K. Senthil and Y. S. Lee, J. Environ. Manage., 2012, 111, 142–149 CrossRef CAS PubMed.
- H. S. Lade, T. R. Waghmode, A. A. Kadam and S. P. Govindwar, Int. Biodeterior. Biodegrad., 2012, 72, 94–107 CrossRef CAS PubMed.
- A. R. Khataee and G. Dehghan, J. Taiwan Inst. Chem. Eng., 2011, 42, 26–33 CrossRef CAS PubMed.
- Y. Yang, B. Wei, Y. Zhao and J. Wang, Bioresour. Technol., 2013, 130, 517–521 CrossRef CAS PubMed.
- F. Vafaei, A. R. Khataee, A. Movafeghi, S. Y. S. Lisar and M. Zarei, Int. Biodeterior. Biodegrad., 2012, 75, 194–200 CrossRef CAS PubMed.
- A. M. Lotito, U. Fratino, G. Bergna and C. Di Iaconi, Chem. Eng. J., 2012, 195–196, 261–269 CrossRef CAS PubMed.
- K. E. Hosseini, M. R. A. Moghaddam and S. H. Hashemi, Int. Biodeterior. Biodegrad., 2012, 71, 43–49 CrossRef PubMed.
- E. H. Koupaie, M. R. Moghaddam and S. H. Hashemi, J. Hazard. Mater., 2011, 195, 147–154 CrossRef PubMed.
- J. Wang, G. F. Liu, H. Lu, R. F. Jin, J. T. Zhou and T. M. Lei, Int. Biodeterior. Biodegrad., 2012, 67, 73–77 CrossRef CAS PubMed.
- L. Huang, G. Sun, T. Yang, B. Zhang, Y. He and X. Wang, Desalination, 2013, 309, 91–96 CrossRef CAS PubMed.
- C. A. Basha, K. V. Selvakumar, H. J. Prabhu, P. Sivashanmugam and C. W. Lee, Sep. Purif. Technol., 2011, 79, 303–309 CrossRef CAS PubMed.
- J. Zhang, L. Wang, G. Zhang, Z. Wang, L. Xu and Z. Fan, J. Colloid Interface Sci., 2013, 389, 273–283 CrossRef CAS PubMed.
- F. I. Hai, K. Yamamoto, F. Nakajima and K. Fukushi, J. Membr. Sci., 2012, 389, 67–75 CrossRef CAS PubMed.
- A. H. Konsowa, H. B. Abd El-Rahman and M. A. Moustafa, Alexandria Eng. J., 2011, 50, 117–125 CrossRef CAS PubMed.
- L. Xu, L. S. Du, C. Wang and W. Xu, J. Membr. Sci., 2012, 409–410, 329–334 CrossRef CAS PubMed.
- I. Vergili, Y. Kaya, U. Sen, Z. B. Gonder and C. Aydiner, Resour., Conserv. Recycl., 2012, 58, 25–35 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |