Structure, magnetism and catecholase activity of the first dicopper(ii) complex having a single μ-alkoxo bridge†
Abstract
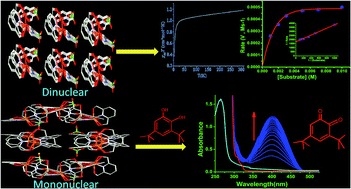
Pyridine-2,6-dimethanol (H2L), which is a versatile donor has a neutral, monoanionic and dianionic coordination behaviour with two different coordination modes viz. tridentate and bidentate towards Cu(II), leading to three different geometric environments around Cu(II) centers. The single crystal X-ray structure reveals two different Cu(II) complexes with H2L: in the first case ClO4− forms a polymer through intermolecular H-bonding, [Cu(H2L)(HL)]ClO4 (1) while the other one is the first report of a mono μ-alkoxo bridged dicopper(II) complex, [Cu(H2L)(HL)Cu(L)]+ (2). The crystallographic asymmetric unit of 2 contains a mononuclear cation [Cu(H2L)(HL)]+ and a dinuclear [Cu(H2L)(HL)Cu(L)]+ unit along with two perchlorate anions, formulated as [Cu(H2L)(HL)][Cu2(H2L)(HL)(L)] (ClO4)2. The magnetic characterization of 2 shows a very weak antiferromagnetic coupling between the Cu(II) centers in the dinuclear unit. This is the first report on the magnetic characterization of the first mono alkoxo-bridged dinuclear Cu(II) complex and, hence needs further examples to firmly establish magneto-structural correlations. The efficient catecholase activity of 2 (kcat = 2.08 × 104 h−1) is attributed to the presence of a mono μ-alkoxo bridge, which holds two Cu(II) centers ∼3.26 Å apart and possibly facilitates the binding of catecholate during the electron transfer reaction. Moreover, the Cu2 center is square planar, and provides one free position for coordination by the substrate molecule.

 Please wait while we load your content...
Please wait while we load your content...