Designing of YVO4 supported β-AgI nano-photocatalyst with improved stability
Abstract
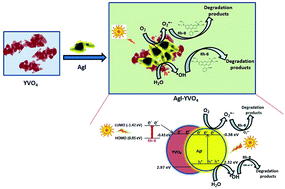
Yttrium ortho vanadate supported β-silver iodide nanoparticles (AgI–YVO4) were designed by a sono-chemical deposition method. The structural, morphological and optical properties of the as-prepared nanoparticles were investigated in detail. Transmission electron microscopy (TEM) results revealed that AgI nano-sheets were attached to YVO4 nano-grains. UV-vis-DRS results indicated that the visible light absorption ability of YVO4 was promoted by AgI modification. The photocatalytic activity was evaluated for the degradation of rhodamine-B (Rh-B) dye under simulated solar light irradiation. The photocatalytic activity of YVO4 was strongly influenced by the concentration of AgI. The photocatalyst of 10% AgI–YVO4 exhibited the best performance (98%) for the degradation of Rh-B. Moreover, the photocatalytic activity was well maintained even after four cycles. The results of this research work would be very useful for the degradation of harmful organic dyes in aqueous environments.

 Please wait while we load your content...
Please wait while we load your content...