Comprehensive chemical secretory measurement of single cells trapped in a micro-droplet array with mass spectrometry†
Abstract
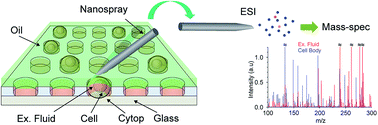
Secretomics, the comprehensive study of cell releasates, offers a non-invasive approach to understanding cell heterogeneity. We here propose single cell small molecule secretomics using micro-droplet arrays and mass spectrometry as a new repertoire of omics technologies. The present method revealed the heterogeneity of secreted small molecules from individual single cells without the use of any invasive processes.

 Please wait while we load your content...
Please wait while we load your content...