Regio- and stereoselective multisubstituted olefin synthesis via hydro/carboalumination of alkynes and subsequent iron-catalysed cross-coupling reaction with alkyl halides†‡
Shintaro
Kawamura§
ab,
Ryosuke
Agata
ab and
Masaharu
Nakamura
*ab
aInternational Research Center for Elements Science (IRCELS), Institute for Chemical Research (ICR), Kyoto University, Uji, Kyoto, 611-0011, Japan
bDepartment of Energy and Hydrocarbon Chemistry, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510, Japan. E-mail: masaharu@scl.kyoto-u.ac.jp
First published on 19th June 2015
Abstract
A new synthetic route towards multisubstituted olefins, which are recurring core units in various pharmaceutical and bioactive compounds, was developed based on the direct cross coupling of alkenylaluminium reagents which were prepared in situ by the hydro- and carbometalation of alkynes, with non-activated alkyl halides in the presence of an iron catalyst. For the first time, alkenylaluminium reagents participated in an iron-catalysed cross-coupling reaction, following the activation of the aluminium reagents by a metal fluoride. The hydro- and carboalumination of alkynes and the subsequent cross-coupling reactions could be conducted in a one-pot manner and proceeded regio- and stereoselectively to give a variety of di-, tri-, and tetrasubstituted alkenes in good to excellent yields.
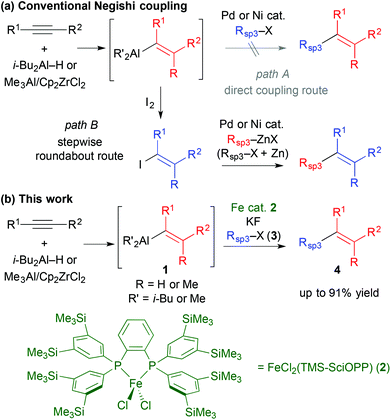
The development of regio- and stereoselective synthetic routes towards multisubstituted olefins, which are recurring core units in various pharmaceutical and bioactive compounds, has been a long-standing challenge in organic chemistry,1 despite the numerous related methodologies that have been described.2 Recently cross-coupling reactions have revealed their utility in olefin synthesis; amply demonstrated by their use in the synthesis of complex natural products.3 A notable expedient approach in this regard is the hydro/carboalumination of alkynes and subsequent cross coupling with organic electrophiles in the presence of Pd or Ni catalysts (the Negishi protocol).4 While this sequential protocol is particularly useful to couple alkenyl sp2 carbon centres with other sp2 carbon electrophiles, it has notable drawbacks. When alkyl (sp3 carbon) electrophiles are employed, competing side reactions, such as β-hydride elimination which is inherently associated with Pd and Ni catalysts,5 impair the cross coupling (Scheme 1a, path A).6 Therefore, the introduction of an alkyl group usually requires a circuitous synthetic strategy: the alkenylaluminium reagent is converted to an alkenyl electrophile, e.g., alkenyl iodide, which is then cross coupled with alkyl zinc reagents in the presence of Pd or Ni catalysts.3 In addition, alkylzinc reagents are typically prepared from alkyl halides with zinc metal (Scheme 1a, path B).7 The direct cross coupling of in situ prepared alkenylaluminium with alkyl halides represents a straightforward method to achieve Csp2–Csp3 bond formation for the synthesis of multisubstituted olefins, which has not been developed using conventional cross-coupling catalysts. We envisaged that iron-catalysed cross coupling of alkyl halides would be feasible for this purpose because of the excellent selectivity and reactivity of iron catalysts in the cross coupling of non-activated alkyl halides, regardless of the presence of β-hydrogens.8–13 Herein, we describe that an iron catalyst enables the direct cross coupling of alkenylaluminium reagents with alkyl halides in the presence of a metal fluoride, and can be applied in the regio- and stereoselective synthesis of multisubstituted olefins (Scheme 1b).
We first examined the iron-catalysed cross coupling of alkenylaluminium reagent 1a, which was prepared by the hydroalumination of 1-octyne with diisobutylaluminium hydride (DIBAL–H) as shown in Scheme 2. Reagent 1a, generated in situ, was reacted with primary alkyl bromide 3a in the presence of a catalytic amount of an Fe–bisphosphine complex, FeCl2(TMS-SciOPP) 2, which was previously reported to act as an efficient catalyst for the cross coupling of arylaluminium reagents.11b Under conditions developed previously for the coupling of arylaluminium reagents, the reaction of 1a with 3a gave the desired product in only 8% yield, albeit with high stereospecificity. In Negishi's original method for the Pd-catalysed cross coupling of alkenylaluminium reagents, it was observed that zinc salts were essential additives or co-catalysts to facilitate the cross coupling, as they form reactive organozinc or organozincate intermediates with the alkenylaluminium species.4a,14 However, the addition of ZnCl2 was totally ineffective here possibly due to the formation of an alkyl (isobutyl) zinc reagent, which may hamper the selective Csp2–Csp3 coupling due to the formation of over-reduced iron species.15
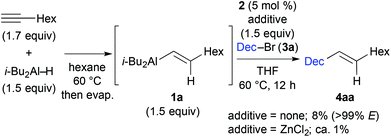 | ||
| Scheme 2 Iron-catalysed cross coupling of 1-bromodecane 3a with alkenylaluminium reagent 1a, prepared by in situ hydroalumination. | ||
These preliminary observations prompted us to identify a new activation method for alkenylaluminium reagents in iron-catalysed cross coupling. Although a variety of reported activation methods are available,16 we envisioned that fluorides or alkoxides could directly activate the organoaluminium reagent via ate complex formation.17 Moreover, we previously demonstrated that organoaluminate species participated efficiently in iron-catalysed cross-coupling reactions.11cTable 1 summarises the results of the additive screening. The addition of NaF, KF, and CsF improved the product yield significantly (entries 2–4), whilst LiF and MgF2 had almost no effect on the reaction (entries 1 and 5). This is possibly due to the strong bonding in the latter metal fluorides. Subsequently, the influence of the counter anion of a range of potassium salts was examined (entries 6–8). The product yield upon addition of KCl was almost the same as that without any additive (entry 6, 12% yield). The addition of t-BuOK decreased the catalytic activity (entry 8).18 When Bu4NF (TBAF) was used, the desired coupling reaction did not proceed (entry 9). Where the cross-coupled product was obtained, the reactions proceeded in a stereospecific manner (entries 1–10), and the addition of KF resulted in the highest yield of the coupling product (entry 3, 76% yield).19 It should be noted that catalyst screening revealed that complex 2 facilitated the reaction most efficiently.20
| Entry | Additive | GC yieldb (%) | RSMb,c (%) | ||
|---|---|---|---|---|---|
| 4aa (E/Z) | Decane (5) | 1-Decane (6) | |||
| a Reactions were conducted for 3 h under the conditions described in Scheme 1. b Yields and ratios of stereoisomers were determined by GC analysis. c Recovery of the starting material 3a. d Isolated yield. e 1-Fluorodecane was obtained in ca. 20% yield. f Run for 12 h. | |||||
| 1 | LiF | 9 (>99% E) | 8 | 12 | 66 |
| 2 | NaF | 38 (>99% E) | 23 | 28 | 5 |
| 3 | KF | 76 (>99% E)d | 9 | 10 | 0 |
| 4 | CsF | 30 (>99% E) | 24 | 15 | 27 |
| 5 | MgF2 | 11 (>99% E) | 8 | 20 | 56 |
| 6 | KCl | 12 (>99% E) | 10 | 23 | 50 |
| 7 | KBr | 31 (>99% E) | 34 | 25 | 5 |
| 8 | t-BuOK | 1 (>99% E) | 0 | 9 | 85 |
| 9e | Bu4NF | 0 | 0 | 8 | 70 |
| 10f | None | 8 (>99% E) | 9 | 18 | 61 |
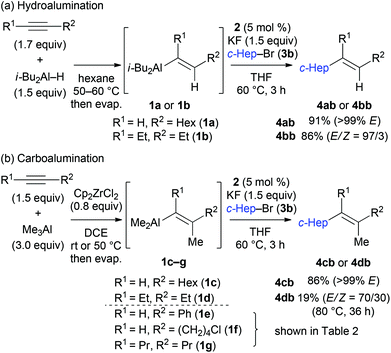
The reactions with substituted alkenylaluminium reagents were examined under the optimised conditions. Substituted alkenylaluminium reagents were prepared in situ by hydroalumination or Zr-mediated carboalumination of terminal and internal alkynes,4a–c and were subsequently used in the iron-catalysed cross coupling with secondary alkyl bromide 3b (Scheme 3). Alkenylaluminium reagents 1a and 1b, prepared by hydroalumination, were readily converted into the desired coupling products 4ab and 4bb in 91% (>99% E) and 86% (E/Z = 97/3) yields respectively (Scheme 3a). Reagents 1c and 1d, formed by carboalumination, also participated in the reaction to afford the desired multisubstituted olefins (86% and 19% yields respectively, Scheme 3b). Fortunately, the presence of concomitant zirconium species in the coupling reaction has no influence on the outcome of the reaction. Rate enhancement by KF was apparent regardless of the structure of the alkenylaluminum reagent used.19 Reagent 1c gave product 4cb with high stereospecificity; however, reagent 1d (E/Z = 80/20) gave product 4db (E/Z = 70/30) with a slightly reduced stereoselectivity. This would be due to E/Z isomerisation of the alkenylaluminum reagent under the coupling conditions.21
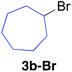
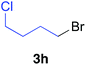
Table 2 illustrates the scope of viable alkyl halide substrates in the coupling reaction. In entries 1–7, the reactivity of a range of primary alkyl halides was examined. 1-Iodo- and 1-bromodecane (3a-I and 3a-Br) gave the desired coupling product 4aa in 73% and 76% yields respectively (entries 1 and 2), whereas the reaction with the corresponding chloride gave 4aa in only 2% yield (entry 3). The reaction of 1-fluorodecane (3a-F) did not proceed at all (entry 4). The results highlight the opposing reactivity profile of the present coupling reaction from that of the Friedel–Craft-type substitution reactions with organoaluminium reagents, where the more electronegative fluoride reacts smoothly.22 Bromo- and chlorocycloheptane were successfully transformed into the desired product 4ab in 91% and 88% yield respectively (entries 5 and 6). An acyclic secondary alkyl chloride also participated in the coupling reaction to afford the product 4ac in 86% yield (entry 7).
| Entrya | Alkyl halide | Product | Isolated yieldb (%) | |
|---|---|---|---|---|
| a See the ESI for details of the reaction conditions for each entry. b The stereoisomeric ratio was determined by GC analysis. c Yield was determined by 1H NMR spectroscopic analysis using 1,1,2,2-tetrachloroethane as an internal standard. | ||||
| 1 |

|
4aa | X = I | 73 (>99% E) |
| 2 | X = Br | 76c (>99% E) | ||
| 3 | X = Cl | 2c (>99% E) | ||
| 4 | X = F | 0 | ||
| 5 |
|
4ab | X = Br | 91 (>99% E) |
| 6 | X = Cl | 88c (>99% E) | ||
| 7 |
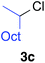
|
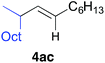
|
86 (>99% E) | |
| 8 |
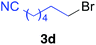
|

|
90 (>99% E) | |
| 9 |
|
|
49 (>99% E) anti/syn = 58/42 | |
| 10 |
|
|
46 (>99% E) | |
| 11 |
|
|
17 (84)c (>99% E) | |
| 12 |
|
|
49 (>99% E) | |
| 13 |
|
|
78 (>99% E) | |
| 14 |
|
|
76 (>99% E) | |
| 15 |
|
|
72 (E/Z = 64/36) | |
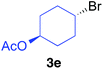
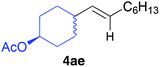
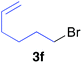
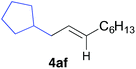
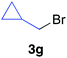
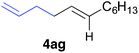
The functional group compatibility of the organoaluminium reagents under the optimized conditions was demonstrated in the reaction of alkyl halides bearing nitrile (3d, 90% yield) and acetoxy groups (3e, 49% yield) as shown in entries 8 and 9. The observed stereochemical scrambling at the reaction centre of 3e could be ascribed to the formation of alkyl radical intermediates, as in related coupling reactions.8–13 Further evidence for a radical intermediate was provided by the reactions of 6-bromo-1-hexene 3f and (bromomethyl)cyclopropane 3g, which resulted in the formation of carbocycle product 4af and ring-opening product 4ag in 46% and 17% yields (84% spectroscopic yield) respectively (entries 10 and 11).
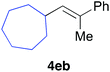
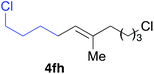
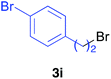
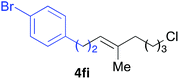
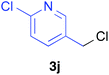
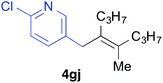
Various alkenylaluminium reagents (1e–g), prepared by Zr-catalysed carboalumination with Me3Al, were subsequently employed in the reaction (entries 12–15). Alkylated (E)-styrene 4eb was obtained in 49% yield from the reaction of β-styrylaluminium 1e (entry 12). Olefins bearing halogens on their side chains can serve as versatile building blocks, and thus the chemoselectivity of the reaction was investigated by comparing substrates containing Csp3–Br, Csp3–Cl, and Csp2–Br moieties; the reaction proceeded selectively at Csp3–Br to provide the desired olefins 4fh and 4fi in 78% and 76% yields respectively (entries 13 and 14). Finally, tetrasubstituted olefin 4gj, bearing a coordinative pyridyl group, was obtained in 72% yield (E/Z = 64/36) (entry 15).21 It should be noted that the chloro group on the pyridyl group remained intact during the coupling reaction, showing again the preference of Csp3–halogen bond cleavage.
In conclusion, we have demonstrated that multisubstituted olefins can be synthesised in a highly regio- and stereoselective manner via alkyne hydro/carboalumination and subsequent iron-catalysed cross coupling between the resulting alkenylaluminum species and non-activated alkyl halides. The use of KF as an additive to activate the alkenylaluminium reagents facilitates the unprecedented direct introduction of various alkyl side chains, thus reducing the steps of olefin synthesis in comparison with the one based on the conventional Negishi protocol. We envisage that the present methodology based on the combination of an organoaluminum reagent and fluoride-promoted iron-catalysed cross coupling will provide facile access to multisubstituted olefins as well as an expedient synthetic method for natural or bioactive complex molecules bearing olefin frameworks.
Acknowledgements
This work was financially supported by the Japan Society for the Promotion of Science (JSPS), through the “Funding Program for Next generation World-Leading Researchers (Next Program)” initiated by the Council for Science and Technology Policy (CSTP), and also by the Japan Science & Technology Agency (JST) through Core Research for Evolutional Science and Technology (CREST 1102545). This work was performed under the Cooperative Research Program of “Network Joint Research Center for Materials and Devices”, the MEXT project of “Integrated Research on Chemical Synthesis”, the Collaborative Research Program of Institute for Chemical Research, Kyoto University. The generous gift of DIBAL from Tosoh Finechem Corporation is acknowledged.Notes and references
- Recent reviews: (a) N. M. G. Franssen, J. N. H. Reek and B. de Bruin, Chem. Soc. Rev., 2013, 42, 5809 RSC; (b) S. Kress and S. Blechert, Chem. Soc. Rev., 2012, 41, 4389 RSC.
- Recent papers: (a) V. Kotek, H. Dvořáková and T. Tobrman, Org. Lett., 2015, 17, 608 CrossRef CAS PubMed; (b) K. P. McGrath and A. Hoveyda, Angew. Chem., Int. Ed., 2014, 53, 1910 CrossRef CAS PubMed; (c) B. Zhou, H. Chen and C. Wang, J. Am. Chem. Soc., 2013, 135, 1264 CrossRef CAS PubMed; (d) Y. Nishihara, M. Suetsugu, D. Saito, M. Kinoshita and M. Iwasaki, Org. Lett., 2013, 15, 2418 CrossRef CAS PubMed; (e) J. Jiao, K. Nakajima and Y. Nishihara, Org. Lett., 2013, 15, 3294 CrossRef CAS PubMed; (f) J. Y. Hamilton, D. Sarlah and E. M. Carreira, J. Am. Chem. Soc., 2012, 135, 994 CrossRef PubMed; (g) L. Ilies, T. Yoshida and E. Nakamura, J. Am. Chem. Soc., 2012, 134, 16951 CrossRef CAS PubMed; (h) C. Wang, T. Tobrman, Z. Xu and E.-i. Negishi, Org. Lett., 2009, 11, 4092 CrossRef CAS PubMed.
- Selected books: (a) A. de Meijere, S. Bräse and M. Oestreich, Metal-Catalyzed Cross-Coupling Reactions and More, Wiley-VCH, Weinheim, 2014 Search PubMed; (b) N. Miyaura, Cross-Coupling Reactions. A Practical Guide, Springer, Berlin, 2002 Search PubMed; (c) M. Schlosser, Organometallics in Synthesis: A Manual, Wiley, London, 2002 Search PubMed; (d) K. Tamao, T. Hiyama and E. Negishi, 30 Years of the Cross-Coupling Reaction, special issue of J. Organomet. Chem, 2002, vol. 653. Selected reviews: (e) X.-F. Wu, P. Anbarasan, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2010, 49, 9047 CrossRef CAS PubMed; (f) E. Negishi, G. Wang, H. Rao and Z. Xu, J. Org. Chem., 2010, 75, 3151 CrossRef CAS PubMed; (g) K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442 CrossRef CAS PubMed.
- Negishi's seminal work and reviews: (a) E. Negishi, N. Okukado, A. O. King, D. E. van Horn and B. I. Spiegel, J. Am. Chem. Soc., 1978, 100, 2254 CrossRef CAS; (b) E. Negishi and S. Baba, J. Chem. Soc., Chem. Commun., 1976, 596 RSC; (c) S. Baba and E. Negishi, J. Am. Chem. Soc., 1976, 98, 6729 CrossRef CAS; (d) E. Negishi, Angew. Chem., Int. Ed., 2011, 50, 6738 CrossRef CAS PubMed; (e) E. Negishi, Z. Huang, G. Wang, S. Mohan, C. Wang and H. Hattori, Acc. Chem. Res., 2008, 41, 1474 CrossRef CAS PubMed; (f) E. Negishi, Bull. Chem. Soc. Jpn., 2007, 80, 233 CrossRef CAS. Selected papers: (g) D. B. Biradar and H.-M. Gau, Org. Biomol. Chem., 2012, 10, 4243 RSC. See also, (h) D. B. Biradar and H.-M. Gau, Chem. Commun., 2011, 10467 RSC; (i) W.-T. Shu, S. Zhou and H.-M. Gau, Synthesis, 2009, 4075 CAS; (j) S.-L. Ku, X.-P. Hui, C.-A. Chen, Y.-Y. Kuo and H.-M. Gau, Chem. Commun., 2007, 3847 RSC; (k) H. Schumann, J. Kaufmann, H.-G. Schmalz, A. Böttcher and B. Gotov, Synlett, 2003, 1783 CrossRef CAS.
- Selected reviews on the cross-coupling reactions of alkyl halides: (a) A. Rudolph and M. Lautens, Angew. Chem., Int. Ed., 2009, 48, 2656 CrossRef CAS PubMed; (b) A. C. Frisch and M. Beller, Angew. Chem., Int. Ed., 2005, 44, 674 CrossRef CAS PubMed; (c) M. R. Netherton and G. C. Fu, Adv. Synth. Catal., 2004, 346, 1525 CrossRef CAS PubMed; (d) D. J. Cárdenas, Angew. Chem., Int. Ed., 2003, 42, 384 CrossRef PubMed; (e) T.-Y. Luh, M.-k. Leung and K. T. Wong, Chem. Rev., 2000, 100, 3187 CrossRef CAS PubMed; (f) D. Cárdenas, Angew. Chem., Int. Ed., 1999, 38, 3018 CrossRef. For the cross-coupling reactions of alkyl metal reagents, see: (g) R. Jana, T. P. Pathak and M. S. Sigman, Chem. Rev., 2011, 111, 1417 CrossRef CAS PubMed.
- Selected review: (a) N. Kambe, T. Iwasaki and J. Terao, Chem. Soc. Rev., 2011, 40, 4937 RSC. Fu reported Pd-catalysed cross-coupling reactions of alkyl halides with organozinc reagents including alkenyl zinc compounds: (b) J. Zhou and G. C. Fu, J. Am. Chem. Soc., 2003, 125, 12527 CrossRef CAS PubMed. Pd-catalysed cross couplings of alkyl halides with alkenyl zirconium compounds, see: (c) S. L. Wiskur, A. Korte and G. C. Fu, J. Am. Chem. Soc., 2004, 126, 82 CrossRef CAS PubMed. See also, Ni-catalysed cross-coupling reactions of alkyl halides with alkenyl metal reagents: (d) S. Lou and G. C. Fu, J. Am. Chem. Soc., 2010, 132, 5010 CrossRef CAS PubMed; (e) X. Dai, N. A. Strotman and G. C. Fu, J. Am. Chem. Soc., 2008, 130, 3302 CrossRef CAS PubMed.
- Selected papers: (a) A. Krasovskiy, V. Malakhov, A. Gavryushin and P. Knochel, Angew. Chem., Int. Ed., 2006, 45, 6040 CrossRef CAS PubMed; (b) R. D. Rieke, M. V. Hanson, J. D. Brown and Q.-J. Niu, J. Org. Chem., 1996, 61, 2726 CrossRef CAS PubMed; (c) L. Zhu, R. M. Wehmeyer and R. D. Rieke, J. Org. Chem., 1991, 56, 1445 CrossRef CAS.
- For selected reviews, see: (a) E. Nakamura, T. Hatakeyama, S. Ito, K. Ishizuka, L. Ilies and M. Nakamura, Org. React., 2014, 83, 1 CAS; (b) K. Riener, S. Haslinger, A. Raba, M. P. Högerl, M. Cokoja, W. A. Herrmann and F. E. Kühn, Chem. Rev., 2014, 114, 5215 CrossRef CAS PubMed; (c) E. Nakamura and N. Yoshikai, J. Org. Chem., 2010, 75, 6061 CrossRef CAS PubMed; (d) W. M. Czaplik, M. Mayer, J. Cvengroš and A. J. von Wangelin, ChemSusChem, 2009, 2, 396 CrossRef CAS PubMed; (e) B. D. Sherry and A. Fürstner, Acc. Chem. Res., 2008, 41, 1500 CrossRef CAS PubMed; (f) A. Fürstner and R. Martin, Chem. Lett., 2004, 33, 624 CrossRef.
- Selected papers on the iron-catalysed cross coupling of alkyl halides with Grignard reagents: (a) C.-L. Sun, H. Krause and A. Fürstner, Adv. Synth. Catal., 2014, 356, 1281 CrossRef CAS PubMed; (b) C. W. Cheung, P. Ren and X. Hu, Org. Lett., 2014, 16, 2566 CrossRef CAS PubMed; (c) M. Guisán-Ceinos, F. Tato, E. Buñuel, P. Calle and D. J. Cárdenas, Chem. Sci., 2013, 4, 1098 RSC; (d) C. Bensoussan, N. Rival, G. Hanquet, F. Colobert, S. Reymond and J. Cossy, Tetrahedron, 2013, 69, 7759 CrossRef CAS PubMed; (e) S. K. Ghorai, M. Jin, T. Hatakeyama and M. Nakamura, Org. Lett., 2012, 14, 1066 CrossRef CAS PubMed; (f) Z. Mo, Q. Zhang and L. Deng, Organometallics, 2012, 31, 6518 CrossRef CAS; (g) S. Meyer, C. M. Orben, S. Demeshko, S. Dechert and F. Meyer, Organometallics, 2011, 30, 6692 CrossRef CAS; (h) M. Jin and M. Nakamura, Chem. Lett., 2011, 40, 1012 CrossRef CAS; (i) Y. Yamaguchi, H. Ando, M. Nagaya, H. Hinago, T. Ito and M. Asami, Chem. Lett., 2011, 40, 983 CrossRef CAS; (j) A. K. Steib, T. Thaler, K. Komeyama, P. Mayer and P. Knochel, Angew. Chem., Int. Ed., 2011, 123, 3361 CrossRef PubMed; (k) T. Hatakeyama, Y. Fujiwara, Y. Okada, T. Itoh, T. Hashimoto, S. Kawamura, K. Ogata, H. Takaya and M. Nakamura, Chem. Lett., 2011, 40, 1030 CrossRef CAS; (l) T. Hatakeyama, Y. Okada, Y. Yoshimoto and M. Nakamura, Angew. Chem., Int. Ed., 2011, 50, 10973 CrossRef CAS PubMed; (m) H.-H. Gao, C.-h. Yan, X.-P. Tao, Y. Xia, H.-M. Sun, Q. Shen and Y. Zhang, Organometallics, 2010, 29, 4189 CrossRef CAS; (n) A. Fürstner, R. Martin, H. Krause, G. Seidel, R. Goddard and C. W. Lehmann, J. Am. Chem. Soc., 2008, 130, 8773 CrossRef PubMed; (o) A. Guérinot, S. Reymond and J. Cossy, Angew. Chem., Int. Ed., 2007, 46, 6521 CrossRef PubMed; (p) M. Nakamura, K. Matsuo, S. Ito and E. Nakamura, J. Am. Chem. Soc., 2004, 126, 3686 CrossRef CAS PubMed; (q) T. Nagano and T. Hayashi, Org. Lett., 2004, 6, 1297 CrossRef CAS PubMed; (r) R. Martin and A. Fürstner, Angew. Chem., Int. Ed., 2004, 43, 3955 CrossRef CAS PubMed; (s) R. B. Bedford, D. W. Bruce, R. M. Frost, J. W. Goodby and M. Hird, Chem. Commun., 2004, 2822 RSC. See also, (t) S. E. Denmark and A. J. Cresswell, J. Org. Chem., 2013, 78, 12593 CrossRef CAS PubMed.
- Selected papers on the reaction with aryl zinc reagents. (a) R. B. Bedford, E. Carter, P. M. Cogswell, N. J. Gower, M. F. Haddow, J. N. Harvery, D. M. Murphy, E. C. Neeve and J. Nunn, Angew. Chem., Int. Ed., 2013, 52, 1285 CrossRef CAS PubMed; (b) X. Lin, F. Zheng and F.-L. Qing, Organometallics, 2012, 31, 1578 CrossRef CAS; (c) T. Hatakeyama, N. Nakagawa and M. Nakamura, Org. Lett., 2009, 11, 4496 CrossRef CAS PubMed; (d) T. Hatakeyama, Y. Kondo, Y. Fujiwara, H. Takaya, S. Ito, E. Nakamura and M. Nakamura, Chem. Commun., 2009, 1216 RSC; (e) R. B. Bedford, M. Huwe and M. C. Wilkinson, Chem. Commun., 2009, 600 RSC; (f) M. Nakamura, S. Ito, K. Matsuo and E. Nakamura, Synlett, 2005, 1794 CrossRef CAS PubMed.
- (a) R. B. Bedford, P. B. Brenner, E. Carter, J. Clifton, P. M. Cogswell, N. J. Gower, M. F. Haddow, J. N. Harvey, J. A. Kehl, D. M. Murphy, E. C. Neeve, M. L. Neidig, J. Nunn, B. E. R. Snyder and J. Taylor, Organometallics, 2014, 33, 5767 CrossRef CAS; (b) S. Kawamura, T. Kawabata, K. Ishizuka and M. Nakamura, Chem. Commun., 2012, 48, 9376 RSC; (c) S. Kawamura, K. Ishizuka, H. Takaya and M. Nakamura, Chem. Commun., 2010, 46, 6054 RSC. Iron-catalysed alkylative intramolecular cross-coupling reaction between a terminal vinyl chloride and terminal olefin moieties with trialkylaluminium was reported. (d) D. Nečas, M. Kotora and I. Císařová, Eur. J. Org. Chem., 2004, 1280 CrossRef PubMed.
- Selected papers on the reaction with organoboron reagents. (a) N. Nakagawa, T. Hatakeyama and M. Nakamura, Chem. Lett., 2015, 44, 486 CrossRef CAS; (b) R. B. Bedford, P. B. Brenner, E. Carter, T. W. Carvell, P. M. Cogswell, T. Gallagher, J. N. Harvey, D. M. Murphy, E. C. Neeve, J. Nunn and D. R. Pye, Chem. – Eur. J., 2014, 20, 7935 CrossRef CAS PubMed; (c) T. Hashimoto, K. K. A. D. S. Kathriarachchi, T. Zenmyo, H. Seike and M. Nakamura, Angew. Chem., Int. Ed., 2012, 51, 8834 CrossRef PubMed; (d) T. Hashimoto, T. Hatakeyama and M. Nakamura, J. Org. Chem., 2012, 77, 1168 CrossRef CAS PubMed; (e) T. Hatakeyama, T. Hashimoto, Y. Kondo, Y. Fujiwara, H. Seike, H. Takaya, Y. Tamada, T. Ono and M. Nakamura, J. Am. Chem. Soc., 2010, 132, 10674 CrossRef CAS PubMed; (f) R. B. Bedford, M. A. Hall, G. R. Hodges, M. Huwe and M. C. Wilkinson, Chem. Commun., 2009, 6430 RSC.
- Selected papers on the mechanistic study on iron-catalysed cross coupling of alkyl halides: (a) H. Takaya, S. Nakajima, N. Nakagawa, K. Isozaki, T. Iwamoto, R. Imayoshi, N. J. Gower, L. Adak, T. Hatakeyama, T. Honma, M. Takagaki, Y. Sunada, H. Nagashima, D. Hashizume, O. Takahashi and M. Nakamura, Bull. Chem. Soc. Jpn., 2015, 88, 410 CrossRef CAS; (b) G. Bauer, M. D. Wodrich, R. Scopelliti and X. Hu, Organometallics, 2015, 34, 289 CrossRef CAS; (c) S. L. Daifuku, M. H. Al-Afyouni, B. E. R. Snyder, J. L. Kneebone and M. L. Neidig, J. Am. Chem. Soc., 2014, 136, 9132 CrossRef CAS PubMed; (d) R. B. Bedford, P. B. Brenner, E. Carter, P. M. Cogswell, M. F. Haddow, J. N. Harvey, D. M. Murphy, J. Nunn and C. H. Woodall, Angew. Chem., Int. Ed., 2014, 53, 1804 CrossRef CAS PubMed; (e) A. Hedström, E. Lindstedt and P.-O. Norrby, J. Organomet. Chem., 2013, 748, 51 CrossRef PubMed; (f) R. Schoch, W. Desens, T. Werner and M. Bauer, Chem. – Eur. J., 2013, 19, 15816 CrossRef CAS PubMed; (g) C. J. Adams, R. B. Bedford, E. Carter, N. J. Gower, M. F. Haddow, J. N. Harvey, M. Huwe, M. A. Cartes, S. M. Mansell, C. Mendoza, D. N. Murphy, E. C. Neeve and J. Nunn, J. Am. Chem. Soc., 2012, 134, 10333 CrossRef CAS PubMed; (h) J. Kleimark, A. Hedström, P.-F. Larsson, C. Johansson and P.-O. Norrby, ChemCatChem, 2009, 1, 152 CrossRef CAS PubMed; (i) D. Noda, Y. Sunada, T. Hatakeyama, M. Nakamura and H. Nagashima, J. Am. Chem. Soc., 2009, 131, 6078 CrossRef CAS PubMed.
- (a) A. Côté and A. B. Charette, J. Am. Chem. Soc., 2008, 130, 2771 CrossRef PubMed; (b) T. Novak, Z. Tan, B. Liang and E. Negishi, J. Am. Chem. Soc., 2005, 127, 2838 CrossRef CAS PubMed. For the importance of zincate or higher order zincate species in the transmetallation step of Negishi coupling, see: (c) L. C. McCann, H. N. Hunter, J. A. C. Clyburne and M. G. Organ, Angew. Chem., Int. Ed., 2012, 51, 7024 CrossRef CAS PubMed; (d) G. T. Achonduh, N. Hadei, C. Valente, S. Avola, C. J. O'Brien and M. G. Organ, Chem. Commun., 2010, 46, 4109 RSC; (e) N. Hadei, E. A. B. Kantchev, C. J. O'Brien and M. G. Organ, Org. Lett., 2005, 7, 3805 CrossRef CAS PubMed.
- While the nature of the active iron species in iron-catalysed cross-coupling reactions is under debate, alkyl nucleophile possessing β-hydrogen is known to reduce iron to lower oxidation states (less than a valency of 0), see: ref. 8d and 9o.
- Selected papers on the activation of organoaluminium reagents. (a) M. Shenglof, D. Gelman, G. A. Molander and J. Blum, Tetrahedron Lett., 2003, 44, 8593 CrossRef CAS PubMed; with carbon nucleophiles. (b) S. Xu, C.-T. Lee, H. Rao and E. Negishi, Adv. Synth. Catal., 2011, 353, 2981 CrossRef CAS PubMed; (c) G. Wang, N. Yin and E. Negishi, Chem. – Eur. J., 2011, 17, 4118 CrossRef CAS PubMed; (d) B. H. Lipshutz and B. Amorelli, J. Am. Chem. Soc., 2009, 131, 1396 CrossRef CAS PubMed; (e) F. Zeng and E. Negishi, Org. Lett., 2001, 3, 719 CrossRef CAS PubMed; with DABCO. (f) V. Conte, G. Fiorani, B. Floris, P. Galloni and S. Woodward, Appl. Catal., A, 2010, 381, 161 CrossRef CAS PubMed; (g) A. Vinogradov and S. Woodward, Org. Synth., 2010, 87, 104 CrossRef CAS; (h) T. Cooper, A. Novak, L. D. Humphreys, M. D. Walker and S. Woodward, Adv. Synth. Catal., 2006, 348, 686 CrossRef CAS PubMed; (i) K. Biswas, A. Chapron, T. Cooper, P. K. Fraser, A. Novak, O. Prieto and S. Woodward, Pure Appl. Chem., 2006, 78, 511 CrossRef CAS; with a chelating ligand on organoaluminum. (j) J. Blum, O. Berlin, D. Milstein, Y. Ben-David, B. C. Wassermann and S. Schutte, Synthesis, 2000, 571 CrossRef CAS PubMed; (k) J. Blum, D. Gelman, E. Shakh, A. Rosenfeld, B. C. Wassermann, M. Frick, B. Heymer, S. Schutte, S. Wernik and H. Schumann, J. Org. Chem., 1997, 62, 8681 CrossRef CAS.
- Selected book: (a) T. Mole and E. A. Jeffery, Organoaluminum Compounds, Elsevier, New York, 1972 Search PubMed. Selected papers: (b) O. Michel, C. Meermann, K. W. Törnroos and R. Anwander, Organometallics, 2009, 28, 4783 CrossRef CAS; (c) H. Lehmkuhl, Angew. Chem., Int. Ed. Engl., 1964, 3, 107 CrossRef PubMed; (d) G. Natta, G. Allegra, G. Perego and A. Zambelli, J. Am. Chem. Soc., 1961, 5033 CrossRef.
- In contrast to our previous report on the rate enhancement by forming aluminum alkoxide species (ref. 11b), alkoxide served as the catalyst poison in this study. Formation of iron-alkoxide species would be favourable due to less Lewis acidity and bulkiness of the alkyl ligand of 2a.
- For investigation on the effect of KF, see ESI.‡.
- For details of the catalyst screening, see ESI.‡.
- J. J. Eisch, R. Amtmann and M. W. Foxton, J. Organomet. Chem., 1969, 16, 55 CrossRef.
- (a) J. Terao, M. Nakamura and N. Kambe, Chem. Commun., 2009, 6011 RSC; (b) M. Ali, L.-P. Liu, G. B. Hammond and B. Xu, Tetrahedron Lett., 2009, 50, 4078 CrossRef CAS PubMed; (c) W. Gu, M. R. Haneline, C. Douvris and O. V. Ozerov, J. Am. Chem. Soc., 2009, 131, 11203 CrossRef CAS PubMed; (d) J. Terao, S. A. Begum, Y. Shinohara, M. Tomita, Y. Naitoh and N. Kambe, Chem. Commun., 2007, 855 RSC; (e) T. Ooi, D. Uraguchi, N. Kagoshima and K. Maruoka, Tetrahedron Lett., 1997, 38, 5679 CrossRef CAS. An efficient iron-catalyzed cross coupling of alkyl fluorides was reported by Deng (ref. 9f).
Footnotes |
| † Dedicated to Prof. E.-i. Negishi on the occasion of his 80th birthday. |
| ‡ Electronic supplementary information (ESI) available: Experimental details, procedures and spectra for characterization. See DOI: 10.1039/c5qo00147a |
| § Present address: RIKEN Center for Sustainable Resource Science, 2-1 Hirosawa, Wako, Saitama, 351-0198, Japan. |
| This journal is © the Partner Organisations 2015 |