 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of bisarylethyne–peptide conjugates†
Martin
Strack
a,
Sina
Langklotz
b,
Julia E.
Bandow
b,
Nils
Metzler-Nolte
 a and
H.
Bauke Albada
*ac
a and
H.
Bauke Albada
*ac
aInorganic Chemistry I, Bioinorganic Chemistry, Faculty of Chemistry and Biochemistry, Ruhr University Bochum, Bochum, Germany
bApplied Microbiology, Faculty of Biology and Biotechnology, Ruhr University Bochum, Bochum, Germany
cInstitute of Chemistry, The Center for Nanoscience and Nanotechnology, The Hebrew University of Jerusalem, Jerusalem 91904, Israel. E-mail: h.b.albada@gmail.com
First published on 13th March 2015
Abstract
Peptide-conjugates with unsaturated carbon chains form attractive molecules that can contribute to both medicinal chemistry and advanced materials sciences. Here, a convenient Fmoc-based solid-phase peptide synthesis method for the preparation of bisarylethyne-conjugates is described. This method allows coupling of an iodo-aryl or aryl-acetylene to resin-bound aryl-acetylene or iodo-aryl containing peptides using catalytic amounts of (PPh3)2PdCl2 and CuI in the presence of DiPEA. The triple bond is reduced quantitatively when 10% silane, either TES or TIS, is present in the acidic cleavage cocktail; deuterated bisarylethylene-species were obtained using deuterated cleavage reagents. Importantly, reduction of the triple-bond is completely suppressed using TFA and phenol as scavengers, and bisarylethyne–peptide conjugates were obtained.
Introduction
Molecules that can interact with both the biological and man-made world attract considerable attention. The peptide-part can interact with many biological processes, and possesses useful structural and functional properties that are applicable in advanced materials and medicinal chemistry.1 Being able to conjugate these molecules to (bio)active (in)organic moieties in a reliable and convenient manner is of utmost importance, and significant progress has been made in this respect. For example, organometallic–peptide conjugates have shown very promising antibacterial properties,2 and the organometallic moiety has contributed to the elucidation of the mode of action of such peptides.3 Conjugation reactions between peptides and (in)organic fragments mostly rely not only on well-established amide-bond forming reactions, but also on carbon–nitrogen bond forming click reactions,4 as well as carbon–carbon cross-coupling reactions, for example the Sonogashira reaction,5 have been used extensively.6 Interestingly, two recently developed silyl-based alkyne-modifying (SAM)-linkers allow convenient preparation of peptides conjugated via their C-terminal carboxamide to compounds that contain an azido-group using the copper-catalysed alkyne–azide cycloaddition reaction.7 Alternatively, an iodo-aryl moiety could be used in a C–C bond forming copper-free Sonogashira cross-coupling reaction.8Apart from C-terminally conjugated peptides, attachment of an (in)organic moiety to a side-chain functionality of a peptide offers more variability than a terminal conjugation. This is of particular importance since most peptide–protein interactions occur via the side-chains of the peptides, and not via one of the two termini.1 For this, specific amino acid side chain functionalities can be addressed, as was shown by the chemoselective metalation of tyrosine residues with a Cp*Rh(III)-fragment.9 Therefore, having the ability to introduce foreign units on the side-chains of amino acid residues is of great value for various applications of peptides.
Here the Sonogashira cross-coupling reaction5 between a peptide that contains an internally positioned iodo-aryl amino acid and an N-terminally positioned arylacetylene unit is applied. The generation of a bisarylethyne moiety may find useful applications in peptide-based materials and in redox-active peptide-conjugates.10 Oligoarylethyne conjugates have been prepared on the resin,11 but their conjugates with peptides are less explored.12 Sonogashira rigidified peptides, that contain an arylethyne unit, have already been studied with respect to their vancomycin- or nisin-mimicking properties,13 and the rigid arylethyne moiety has been applied as a rigid spacer between the two bioactive ends of an ITAM-based ligand that binds to the Syk tandem SH2 domain.14 However, solid-phase methods to obtain these constructs are limited, and a reliable procedure to obtain bisarylethyne-bridged peptides of which the bisarylethyne-forming reaction was performed on the resin was not available. In this paper, it is shown how triple bond bridged peptides can be obtained. Furthermore, a mild procedure for the reduction of bisarylethyne-bridged peptides to bisarylethylene-bridged peptides during the cleavage step when a silane-scavenger is present in the cleavage mixture is presented.
Results and discussion
The study was initiated with a dipeptide test-compound. First, the conditions that would be most suitable for the installation of a bisarylethyne unit on a peptide were determined. In view of previous observations that arylacetylene groups hydrate under the acidic conditions that are required to simultaneously deprotect amino acid side chain functionalities and remove the peptide from the resin,15 it was envisioned that the even more electron-rich bisarylethyne moiety could undergo a similar (unwanted) side-reaction. Once conditions that produce the desired bisarylethyne-bridge were uncovered, the study was extended to peptides (vide infra).Thus, a resin-bound dipeptide (1) was prepared that contained a 4-iodophenylalanine residue using standard Fmoc-based peptide synthesis (Scheme 1). A Sonogashira cross-coupling reaction between peptide 1 and an arylacetylene moiety, i.e. phenylacetylene (2a) or 2-ethynylpyridine (2b), was performed using (PPh3)2PdCl2 and CuI in the presence of DiPEA. This provided resin-bound bisarylacetylene containing dipeptides 3a or 3b. Cleavage of this bisphenylethyne-containing product was initially performed using TFA–TIS–H2O (95/2.5/2.5, v/v/v), which is a standard cocktail used for the cleavage of peptides from a resin.16 This provided a mixture of inseparable products that contained non-reduced and fully reduced triple-bonds.17 Since the reduction could not be avoided when a silane was present, we attempted to drive the reduction of the triple bond to completion by adding more silane, and by using the more reactive TES. We reasoned that although the triple bond forms an attractive rigidifying feature with interesting opto- and electrochemical properties, having the ability to fully reduce the triple bond would not only result in the formation of valuable compounds for control experiments as it produces non-conductive peptides with a higher flexibility in the bridging section, it would also help us to gain insight into the source of reduction, and how to avoid it. Therefore, using TFA–TES–H2O (80/10/10, v/v/v) we obtained the fully reduced dipeptide 4 in acceptable yields after purification. Importantly, a partially reduced peptide was not observed using this method.
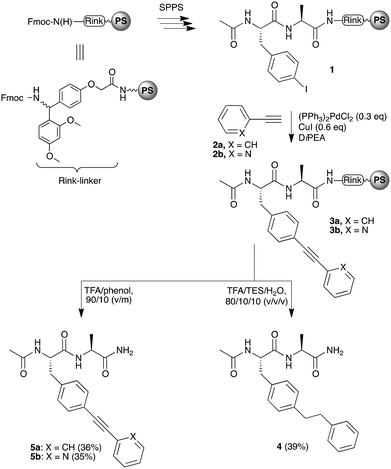 | ||
| Scheme 1 Synthesis of resin-bound bisphenylethyne-containing dipeptide 3, and its cleavage-reduction from the resin to 4, or non-reducing cleavage from the resin to 5a and 5b. | ||
Since our main interest was in the triple-bond containing bisarylethyne dipeptide, i.e.5a or 5b (vide infra), we wanted to obtain more insight into the reduction process, and how this could be avoided. For this, reagents of which the reactive groups were deuterated, i.e. d-TFA, d-TES, and D2O, were used (Scheme 2). In the presence of three deuterated reagents, reduction of the triple bond resulted in tetra-deuterated species 8, which was obtained in purified yields that were similar to the reduction with hydrogen containing reagents (as in 4). However, when a mixture of deuterated reagents was used, various levels of deuteration were detected, ranging from non-deuterated to tetra-deuterated species (Chart 1). By means of the intensity of the peaks in ESI-MS spectra of the formed species, we were able to determine the ratio of deuteration, which could then be correlated with the reagent of the cleavage mixture that was mostly involved in the reduction. For example, when normal TFA and H2O together with d-TES was used, di- and tri-deuterated species dominated the spectrum, with a total of 74%. However, when normal TES and d-TFA and D2O were used, mono- and di-deuterated species were mostly present, with a total of 79%. Considering that the ratio of TFA–TES–water was 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v/v), and the observation that peptide 6, which was exposed to only 10% deuterated species, was more deuterated than peptide 7, which was exposed to 90% deuterated species, it became clear that the main contributor to the reductive deuteration of the triple bond is the silane. However, the fact that a range of deuterated species is obtained indicates that several mechanisms occur during this reduction.
10 (v/v/v), and the observation that peptide 6, which was exposed to only 10% deuterated species, was more deuterated than peptide 7, which was exposed to 90% deuterated species, it became clear that the main contributor to the reductive deuteration of the triple bond is the silane. However, the fact that a range of deuterated species is obtained indicates that several mechanisms occur during this reduction.
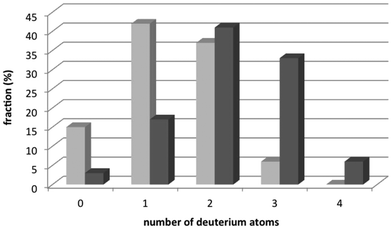 | ||
| Chart 1 Extent of deuteration of bisarylethyne peptide 3a by means of deuterated cleavage reagents. The dark-grey bars represent peptide 6, and the light-grey bars represent peptide 7. | ||
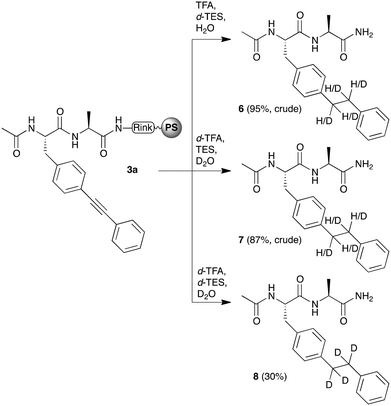 | ||
| Scheme 2 Reduction of the triple bond of a resin-bound bisarylethyne-containing peptide (3a) by means of partially deuterated mixtures of TFA–TES–water – 80/10/10 (v/v/v). | ||
From the literature it is known that the triple bond of bisarylethyne moieties can be reduced using TES, but this usually requires the presence of a (late) transition metal(ion) as the catalyst. This reaction typically results in the formation of E-18 or Z-alkenes,19 or various hydrosilated products;20 only with a large excess of TES and in the presence of HCl and AlCl3 a complete reduction was described.21 Apparently, the reduction also proceeds smoothly with an excess of TFA and only 10% of the silane.
Having established that the silane is the major contributor to the reduction of the triple bond, we explored cleavage mixtures that would keep the bisarylethyne-unit intact. Using a mixture that contained only TFA and water, only a more polar species was observed by HPLC analysis, and ESI-MS analysis of that species indicated the formation of a hydrated species, supposedly an asymmetric ketone that was formed by hydration of the triple bond (data not shown). Fortunately, when phenol was used instead of silane and water, reduction of the triple bond was completely avoided, and the desired bisarylethyne unit remained intact (see also Scheme 1, compounds 5a and 5b). The desired bisarylethyne-containing dipeptide 5a was obtained in appropriate yields; a pyridine-containing construct (5b) was also obtained using this cleavage mixture. This latter conjugate is particularly interesting as a coordination partner to a redox-active metal-complex with catalytic or medicinal properties.
After establishing the conditions by which bisarylethyne–peptide conjugates could be obtained, the preparation of bisarylethyne-bridged peptides was pursued. For this, we prepared a resin-bound Gly-Tyr-Val-Ser tetrapeptide of which the N-terminus was derivatized with 4-ethynylbenzoic acid (9) (Scheme 3). To the terminal acetylene-moiety an aryl-iodide was coupled, either Fmoc-(4-iodophenylalanine) or Ac-(4-I)Phe-Ser-NH2 (11), using the established Sonogashira cross-coupling conditions. The Fmoc-group of the attached Fmoc-(4-iodophenylalanine) was removed using piperidine before the resulting bisarylethyne-bridged constructs were cleaved from the resin using TFA–phenol, which also removed the tert-butyl protecting groups. Both purified constructs were obtained in acceptable yields, i.e. 25% and 22% for constructs 10 and 12, respectively. HPLC analysis of the crude mixture only revealed the presence of trace impurities, with 68% and 60% purity of peptides 10 and 12, respectively; the parent-peptide that would result from cleaved unreacted peptide 9 was not observed. Considering that these constructs were obtained in 13 or 12 steps, respectively, yields of approximately 90% per step are achieved.
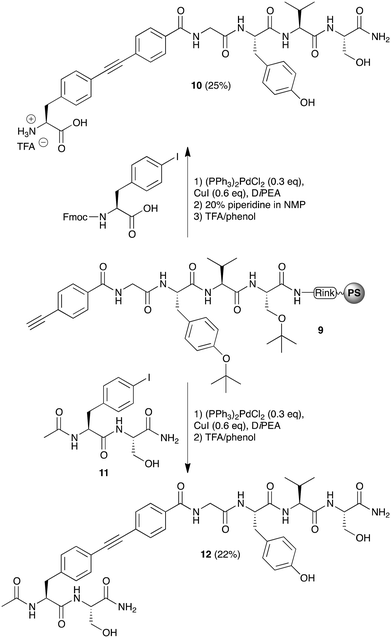 | ||
| Scheme 3 Synthesis of bisarylethyne-bridged peptides 10 and 12 using the Sonogashira cross-coupling reaction. | ||
Experimental
Typical procedure for the synthesis of bisarylethyne-containing peptides
Peptides were prepared using standard Fmoc-based solid-phase peptide synthesis procedures. The Sonogashira cross-coupling reaction was performed using dry peptide-containing resin, which contained either an iodo-aryl or arylacetylene derivatized peptide. The resin was placed in a dry Schlenk flask and DMF, DiPEA, and either the arylacetylene or iodo-aryl reaction partner were added. The reaction mixture was degassed using nitrogen (15 min) before (PPh3)2PdCl2 and CuI were added. The suspension was stirred overnight at room temperature. After this, the reaction mixture was filtered from the resin, and the resin was repeatedly washed with DCM, DMF, DCM, and Et2O. The peptide-conjugates were cleaved from the resin using TFA–phenol (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/w) for 60 min. The resulting cleaved and deprotected peptides were filtered from the resin, and precipitated in cold Et2O after the TFA-solution was concentrated. Crude bisarylethyne–peptide conjugates were purified by preparative HPLC, and the desired products were obtained by lyophilization of the collected fractions.
1, v/w) for 60 min. The resulting cleaved and deprotected peptides were filtered from the resin, and precipitated in cold Et2O after the TFA-solution was concentrated. Crude bisarylethyne–peptide conjugates were purified by preparative HPLC, and the desired products were obtained by lyophilization of the collected fractions.
Conclusions
A reliable method for the synthesis of bisarylethyne-containing peptides is developed using standard Fmoc-based solid-phase peptide synthesis procedures, and a heterogeneous Sonogashira cross-coupling reaction between a resin-bound and a solution-based reaction partner. Coupling of arylacetylenes or iodo-aryl compounds to resin-bound iodo-aryl and arylacetylene derivatized peptides is shown. Cleavage of the bisarylethyne-containing peptide using TFA and phenol provides the desired triple-bond containing conjugate species, whereas the presence of TES or TIS results in the reduction of the triple bond to the ethylene-moiety. Using deuterated solvents, partially or fully deuterated bisarylethylene-units can be obtained. Due to the potentially interesting redox- and photochemical properties of these bisarylethyne units, we anticipate that more elaborate hybrid-molecules can find applications in both medicinal chemistry and advanced materials.Acknowledgements
This research was supported by the ‘Fonds der Chemischen Industrie’ (fellowship to M.S.), the German Federal State of North Rhine-Westphalia (NRW), Germany, and the European Regional Development Fund, ‘Investing in your Future’. J.E.B. acknowledges funding from NRW for a grant (Translation of Innovative Antibiotics – TinA) and the high resolution mass spectrometer.Notes and references
- A. J. Kastin, Handbook of Biologically Active Peptides, Elsevier Inc., Burlington, MA, 1st edn, 2006 Search PubMed.
- H. B. Albada, P. Prochnow, S. Bobersky, J. E. Bandow and N. Metzler-Nolte, Chem. Sci., 2014, 5, 4453 RSC.
- M. Wenzel, A.-I. Chiriac, A. Otto, D. Zweytick, C. May, C. Schumacher, R. Gust, H. B. Albada, M. Penkova, U. Krämer, R. Erdmann, N. Metzler-Nolte, S. K. Straus, E. Bremer, D. Becher, H. Brötz-Oesterhelt, J.-G. Sahl and J. E. Bandow, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, E1409 CrossRef CAS PubMed.
- (a) C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef PubMed; (b) M. Meldal and C. W. Tornøe, Chem. Rev., 2008, 108, 2952 CrossRef CAS PubMed.
- K. Sonogashira, Y. Tohda and N. Hagihara, Tetrahedron Lett., 1975, 50, 4467 CrossRef.
- (a) U. Hoffmanns and N. Metzler-Nolte, Bioconjugate Chem., 2006, 17, 204 CrossRef CAS PubMed; (b) H. Pfeiffer, A. Rojas, J. Niesel and U. Schatzschneider, Dalton Trans., 2009, 4292 RSC; (c) O. Brosch, T. Weyhermüller and N. Metzler-Nolte, Eur. J. Inorg. Chem., 2000, 2000, 323 CrossRef; (d) O. Brosch, T. Weyhermüller and N. Metzler-Nolte, Inorg. Chem., 1999, 38, 5308 CrossRef CAS.
- (a) M. Strack, S. Langklotz, J. E. Bandow, N. Metzler-Nolte and H. B. Albada, J. Org. Chem., 2012, 77, 9954 CrossRef CAS PubMed; (b) M. Strack, N. Metzler-Nolte and H. B. Albada, Org. Lett., 2013, 15, 3126 CrossRef CAS PubMed.
- M. Strack, N. Metzler-Nolte and H. B. Albada, Synthesis, 2014, 46, 2293 CrossRef CAS PubMed.
- (a) H. B. Albada, F. Wieberneit, I. Dijkgraaf, J. H. Harvey, J. L. Whistler, R. Stoll, N. Metzler-Nolte and R. H. Fish, J. Am. Chem. Soc., 2012, 134, 10321 CrossRef CAS PubMed; (b) F. Wieberneit, A. Korste, H. B. Albada, N. Metzler-Nolte and R. Stoll, Dalton Trans., 2013, 42, 9799 RSC.
- See for a ground-breaking study on the conductive nature of the bisphenylethyne-unit: L. A. Bumm, J. J. Arnold, M. T. Cygan, T. D. Dunbar, T. P. Burgin, L. Jones III, D. L. Allara, J. M. Tour and P. S. Weiss, Science, 1996, 271, 1705 Search PubMed And for various applications: U. H. F. Bunz, Macromol. Rapid Commun., 2009, 30, 772 CrossRef CAS PubMed.
- (a) V. Brun, M. Legraverend and D. S. Grierson, Tetrahedron Lett., 2001, 42, 8169 CrossRef CAS; (b) N. F. Utesch and F. Diederich, Org. Biomol. Chem., 2003, 1, 237 RSC; (c) A. C. Spivey, J. McKendrick and R. Srikaran, J. Org. Chem., 2003, 68, 1843 CrossRef CAS PubMed; (d) J.-J. Hwang and J. M. Tour, Tetrahedron, 2002, 58, 10387 CrossRef CAS.
- See for example: U. Hoffmanns and N. Metzler-Nolte, Bioconjugate Chem., 2006, 17, 204–213 CrossRef CAS PubMed.
- (a) N. Ghalit, D. T. S. Rijkers and R. M. J. Liskamp, J. Mol. Catal. A: Chem., 2006, 254, 68 CrossRef CAS PubMed; (b) N. Ghalit, A. J. Poot, A. Fürstner, D. T. S. Rijkers and R. M. J. Liskamp, Org. Lett., 2005, 7, 2961 CrossRef CAS PubMed; (c) H. T. ten Brink, D. T. S. Rijkers and R. M. J. Liskamp, J. Org. Chem., 2006, 71, 1817 CrossRef CAS PubMed.
- N. J. de Mol, M. I. Catalina, F. J. Dekker, M. J. E. Fischer, A. J. R. Heck and R. M. J. Liskamp, ChemBioChem, 2005, 6, 2261 CrossRef CAS PubMed.
- (a) S. T. Le Quement, T. E. Nielsen and M. Meldal, J. Comb. Chem., 2008, 10, 546 CrossRef CAS PubMed; (b) B. Meseguer, D. Alonso-Díaz, N. Griebenow, T. Herget and H. Waldmann, Angew. Chem., Int. Ed., 1999, 38, 2902 CrossRef CAS.
- That the reduction strongly depends on the electronic nature of the bisarylethyne-unit, i.e. occurs with electron-rich units, is shown in the application of a Tolan amino acid (which is –C(O)NH–Ar–C
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–Ar–C(O)NH–), which was not reduced when treated with TFA–TIS–H2O – 95/2.5/2.5 (v/v/v) see: D. A. Offermann, J. E. McKendrick, J. J. P. Sejberg, B. Mo, M. D. Holdom, B. A. Helm, R. J. Leatherbarrow, A. J. Beavil, B. J. Sutton and A. C. Spivey, J. Org. Chem., 2012, 77, 3197 CrossRef CAS PubMed.
C–Ar–C(O)NH–), which was not reduced when treated with TFA–TIS–H2O – 95/2.5/2.5 (v/v/v) see: D. A. Offermann, J. E. McKendrick, J. J. P. Sejberg, B. Mo, M. D. Holdom, B. A. Helm, R. J. Leatherbarrow, A. J. Beavil, B. J. Sutton and A. C. Spivey, J. Org. Chem., 2012, 77, 3197 CrossRef CAS PubMed. - See for a similar outcome on arylacetylene-peptides: S. T. Le Quement, T. E. Nielsen and M. Meldal, J. Comb. Chem., 2008, 10, 546 CrossRef CAS PubMed.
- F. Luo, C. Pan, W. Wang, Z. Ye and J. Cheng, Tetrahedron, 2010, 66, 1399 CrossRef CAS PubMed.
- Y. M. A. Mohamed and T. V. Hansen, Tetrahedron, 2013, 69, 3872 CrossRef CAS PubMed.
- N. Asao, T. Sudo and Y. Yamamoto, J. Org. Chem., 1996, 61, 7654 CrossRef CAS.
- G. L. Larson and J. L. Fry, Ionic and Organometallic-Catalyzed Organosilane Reductions, in Organic Reactions, Hoboken, NJ, USA, 2008, vol. 71, pp. 1–737 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General information, detailed experimental procedures, ESI-MS and HR-MS data, 1H and 13C NMR-spectra, and HPLC-traces of all new compounds. See DOI: 10.1039/c4qo00357h |
| This journal is © the Partner Organisations 2015 |
