Facile fabrication of InSe nanosheets: towards efficient visible-light-driven H2 production by coupling with P25†
Ding
Wei
,
Lihua
Yao
,
Song
Yang
,
Jufang
Hu
,
Minhua
Cao
* and
Changwen
Hu
*
Key Laboratory of Cluster Science, Ministry of Education of China, Beijing Key Laboratory of Photoelectronic/Electrophotonic Conversion Materials, Department of Chemistry, Beijing Institute of Technology, Beijing 100081, P. R. China. E-mail: caomh@bit.edu.cn; cwhu@bit.edu.cn; Fax: +86-10-68912631; Tel: +86-10-68918468
First published on 1st June 2015
Abstract
A cubic InSe nanosheet photocatalyst has been successfully synthesized via a hydrothermal process followed by a calcination treatment, and it was for the first time used for visible light photocatalytic H2 production. Its photoactivity was further improved by coupling with P25, which was attributed mainly to the higher efficiency of charge separation and transfer in the InSe–rutile–anatase surface junctions.
Solar energy utilization is a sustainable strategy to ultimately solve the problems of fossil fuel shortage and environmental pollution, while semiconductor based photocatalytic water splitting for hydrogen production has been considered as a promising and green route for the efficient utilization of solar energy.1 Among various reported semiconductors, TiO2 is the most studied semiconductor as an efficient photocatalyst due to its appropriate electronic band structure, environmental friendliness, low cost and high stability.2 However, TiO2 photocatalysis has been limited to ultraviolet (UV) light instead of the more abundant visible light due to its wide band gap (3.0–3.2 eV). Besides TiO2, CdS, Pt nanowires/Ni(OH)2, etc. also exhibit superior catalytic activity for H2 evolution reaction.3 Therefore, developing visible-light-responsive photocatalysts has become one of the most important topics in photocatalytic hydrogen production research today.
As compared to their wide bandgap counterparts, metal selenides, such as CdSe, are attractive photocatalytic materials which can be widely used in visible-light-driven H2 production due to their narrow band gap.4 However, toxic Cd2+ is left in the liquid after irradiation from the photodecomposition of CdSe.5 Hence, the other metal selenides like NiSe and In2Se3 are good candidates instead of toxic CdSe.6 In our previous study, porous γ-In2Se3 tetragonum was successfully synthesized and used for hydrogen production under UV and visible light irradiation.6b As the photocatalytic efficiency of a single selenide photocatalyst is limited by its photocorrosion and the rapid recombination of photogenerated electrons and holes, great efforts should be devoted to the development of a rational design for the fabrication of a selenide heterostructure system in which the photogenerated carriers can be transferred to different counterparts thus promoting the photoactivity.4a,b
As an important III–VI group semiconductor, hexagonal phase InSe with a layered structure has attractive applications in solar energy conversion due to its well-known optical and electrical properties, particularly controllable band-gap energy in the full visible spectral range. There have been several reports on hexagonal phase InSe-based electrical devices, optoelectronic devices and on their photoelectrochemical properties.7 Although hexagonal phase InSe have been obtained by several methods, such as chemical vapor deposition and the electrochemical technique, it is difficult to produce single phase and stoichiometric InSe due to the co-existence of several phases of the In–Se system like InSe, In2Se3 and In6Se7.8 In addition, hexagonal phase InSe obtained by the conventional process are general bulk materials, and a few are InSe nano-materials with a regular shape due to the harsh synthetic conditions.9
Besides hexagonal phase, InSe also has a cubic phase, which is quite rare both in the bulk state and in nanosized materials.10 As the optical and electrical properties are size- and shape-dependent, great effort has been focused on the phase structure and morphology-selective fabrication of this material. Therefore, it is a vital concern to develop a new and convenient method to synthesize single-phase InSe nanostructures. In particular, it is still rather challenging to synthesize cubic phase InSe nanostructures.
Recently, Qian et al. reported a surfactant assisted hydrothermal approach to prepare size and shape controlled nanostructures, such as CTAB-assisted synthesis of bundle-like Te nanorods, SDBS-assisted synthesis of Cu nanowires and Ni nanowires, SDS-assisted synthesis of nickel film and uniform PbS nanorod bundles, PVP-assisted synthesis of NiSe2 tubular microcrystals and so on.11 In this study, we firstly simulate this synthetic strategy to prepare cubic phase InSe nanosheets and InSe–TiO2 (anatase and rutile) heterostructures. We also investigated the modification effects of TiO2 (anatase and rutile) on the visible light-driven photocatalytic H2 production activity of the cubic phase InSe nanosheets. Experimental results demonstrated that the as-prepared InSe–TiO2 (anatase and rutile) photocatalyst showed drastically enhanced photocatalytic H2 production from an aqueous ascorbic acid (AA) solution compared to that of pure InSe and InSe–TiO2 (anatase), indicating that the synergetic effect of mix-phase TiO2 nanoparticles (NPs) played an important role in the photocatalytic H2 production rate.
The InSe nanosheets were synthesized by a facile two-step hydrothermal–calcining process (shown in Scheme 1), which is an effective method to synthesize single phase photocatalysts.12 Firstly, a yellow In–Se-based precursor was hydrothermally synthesized by using sodium dodecylbenzyl sulfate (SDBS) as a surfactant. It was believed that the surfactant SDBS might play a role in preventing the aggregation of precursor nanoparticles in the initial stage of nanosheet growth and kinetically controlling the growth rates of various crystallographic facets.11b Subsequently, the precursor was calcined under an N2 atmosphere at 250 °C, then it turned into red color InSe nanosheets. The preparation of InSe–TiO2 (anatase and rutile) heterostructures was similar to that of InSe nanosheets, and P25 was added during the hydrothermal step. The detailed synthesis process of both InSe and InSe–TiO2 heterostructures is described in the ESI.†
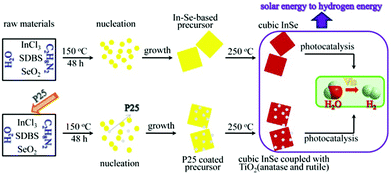 | ||
| Scheme 1 Formation paths of cubic InSe and InSe–TiO2. They were used for photocatalytic H2 production. | ||
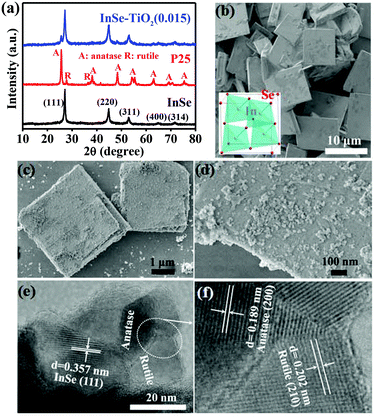
The phase composition of as-prepared samples was first investigated by X-ray powder diffraction (XRD). As shown in Fig. 1a (black curve), all diffraction peaks in the XRD pattern for the sample obtained in the absence of P25 match well with the cubic InSe phase, and no characteristic peaks from impurities were detected.10 When 0.015 g P25 was used, the resulting sample was made up of cubic InSe and TiO2 (Fig. 1a, blue curve), which was named InSe–TiO2(0.015), suggesting the fact that InSe is coupled with P25.
Fig. 1b presents a scanning electron microscopy (SEM) image of the InSe sample. Clearly, the InSe sample is composed of square-like nanosheets with side lengths in the range of 5–10 μm and a thickness of about 100 nm. When SDBS was replaced by other surfactants, such as, CTAB, SDS and PVP, or when no surfactant was used, pure InSe could still be obtained, but the morphologies of the products were irregular (Fig. S1 and S2†). The explanation for this probably indicates that these surfactants were different in the carbon chain length and the hydrophilic group, and thus they were alien in modulating the growth kinetics of InSe crystals. Fig. 1c displays the SEM image of the InSe–TiO2(0.015) sample, from which it can be seen that this sample also exhibits a nanosheet shape. However, different from the above mentioned pure InSe sample, the nanosheets are coated by TiO2 NPs with an average diameter of ca. 10 nm, demonstrating the successful synthesis of InSe–TiO2 heterostructures (Fig. 1d). Obviously, the introduction of TiO2 phase into InSe does not change their respective morphology. The high-resolution TEM (HRTEM) images (Fig. 1e and f) of the InSe–TiO2(0.015) sample further demonstrate its microstructure. There are three types of lattice spacings, 0.357, 0.189, and 0.202 nm, which can be indexed to the (111) plane of cubic InSe, the (200) plane of anatase TiO2, and the (210) plane of rutile TiO2, respectively. This result confirms that the hydrothermal treatment has no influence on the phase composition of P25.
X-ray photoelectron spectroscopy (XPS) measurements were carried out to determine the surface compositions and chemical states of the as-obtained samples. As clearly disclosed by Fig. 2a, the high-resolution In 3d XPS spectra of the InSe sample show two typical In 3d5/2 and In 3d3/2 peaks centered at 444.6 eV and 452.2 eV, respectively, assigned to the In–Se bond.13 For the InSe–TiO2(0.015) heterostructure, the In 3d5/2 and In 3d3/2 peaks are slightly shifted to a lower binding energy of 444.3 eV and 451.9 eV, respectively, indicating that there exists electronic coupling between InSe and TiO2.14 The possible reason may be that the electronegativity of Ti is smaller than that of In, which causes the increase of electron density around the In atom. Therefore, the presence of titanium species in InSe–TiO2(0.015) decreases the binding energies of In 3d. The XPS results are in good agreement with those from HRTEM described above.
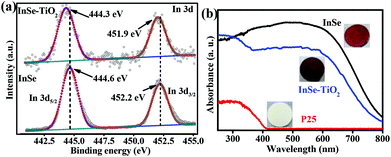 | ||
| Fig. 2 (a) High-resolution XPS spectra of In 3d from InSe and InSe–TiO2(0.015); (b) UV-vis absorption spectra and photographs of InSe, InSe–TiO2(0.015) and P25. | ||
UV-visible absorption spectra for as-prepared samples as well as P25 are shown in Fig. 2b. Obviously, the InSe sample can absorb visible light with a band gap of 1.5 eV, while P25 exhibits only UV absorption with a band gap of 3.0 eV. Compared with pristine InSe and P25, the InSe–TiO2(0.015) heterostructure exhibits in-between absorption with a band gap of 1.6 eV, which may result from the synergistic effect between the host InSe nanosheets and the guest P25 NPs in the InSe–TiO2 heterostructure. Furthermore, the absorption of InSe–TiO2 samples in the visible light region decreases with the increase of P25 dosage (Fig. S4b†).
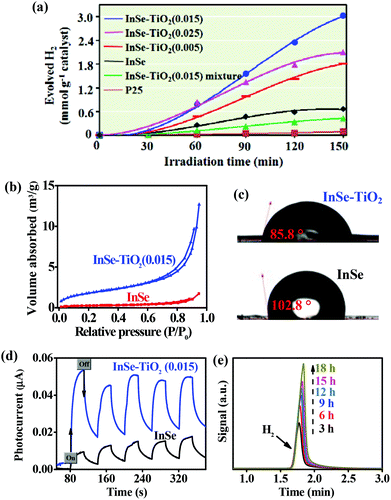
In view of the excellent visible light absorption of InSe and InSe–TiO2(0.015), we further studied their photocatalytic H2 production under visible light irradiation (λ > 420 nm, Fig. S3†). It is widely believed that an appropriate electron donor used in the solution/semiconductor suspension contributes to high photocatalytic activity.15 Hence, in our experiments, ascorbic acid (AA) was used as a sacrificial reagent. Fig. 3a displays the photocatalytic H2 production rates of different InSe–TiO2 (anatase and rutile) heterostructures and other reference samples under visible light irradiation. As expected, the InSe–TiO2 heterostructures exhibited better photocatalytic activity than the InSe nanosheets, P25 and the InSe–TiO2 mixture. Among all InSe–TiO2 (anatase and rutile) heterostructures, the InSe–TiO2(0.015) sample has the maximum H2 production rate of 1.1 mmol (g catalyst h)−1. In this regard, the photocatalytic activity of InSe–TiO2(0.015) exceeds that of pure InSe nanosheets by a factor of 4.1, which is significantly higher than that of most metal selenides and TiO2 photocatalysts.4,6,16 However, a further increase in TiO2 content leads to a deterioration in photocatalytic activity (Fig. 3a and S4a†). It is reasonable because excessive TiO2 modification led to the shielding of the InSe nanosheet surface, thus rapidly decreasing its visible light harvesting ability, as proved in Fig. S4b and S5b.† Moreover, to further confirm that co-existing anatase and rutile TiO2 particles as well as the heterojunctions formed between TiO2 and InSe have an important role in the enhanced photocatalytic performance, we also studied the time-dependent photocatalytic hydrogen evolution of the InSe–TiO2 mixture and InSe–TiO2(A) samples (Fig. 3a and S6†). Obviously, the H2 production rates of both samples are much lower than those of InSe–TiO2(0.015) heterostructures. In addition, the H2 production rate (1.1 mmol (g h)−1) of the InSe–TiO2(0.015) sample is higher than that of most of the reported catalysts (Table S1†).
Compared to pure InSe nanosheets, the efficient visible-light-driven photocatalytic activity of InSe–TiO2(0.015) heterostructures can be attributed to the following three factors: (1) as shown in Fig. 3b, the InSe–TiO2(0.015) heterostructure has a larger specific surface area (0.9 m2 g−1 for InSe and 6.8 m2 g−1 for the InSe–TiO2(0.015) heterostructure), which can offer more active adsorption sites for photocatalytic reaction and thus favors an enhanced H2 production rate.17 (2) The surface hydrophilicity of InSe nanosheets is evidently improved by modification with TiO2 NPs, which is confirmed by the fact that the contact angle (CA) of InSe–TiO2(0.015) is smaller than that of InSe nanosheets (Fig. 3c). This can result in better interfacial charge separation and consequent water reduction efficiency.18 (3) The junctions formed among InSe, rutile and anatase (Fig. 1e and f) favor the fast charge separation at the interface due to the proper band alignment.19 The higher separation and transfer efficiency of charges in InSe–TiO2(0.015) has been further confirmed by the photocurrent response test (Fig. 3d).
To optimize the photocatalytic reaction conditions, the effect of the concentrations of ascorbic acid and Pt on the H2 evolution rate catalyzed by InSe–TiO2(0.015) was examined. As shown in Fig. S7 and S8,† when the concentration of ascorbic acid was 0.075 mol L−1 and with 2.5% Pt loaded, the H2 production rate reached the highest value of 1.5 mmol (g catalyst h)−1 with apparent quantum efficiency of 25.5% at 450 nm. Moreover, the InSe–TiO2(0.015) catalyst can go through a continuous long-time photoreaction under the optimized conditions, without evident changes in the H2 evolution rate (Fig. 3e). The total mole of produced H2 (1.03 mmol) significantly exceeded that of the photocatalyst (0.23 mmol), confirming the photocatalytic behavior of InSe–TiO2(0.015).
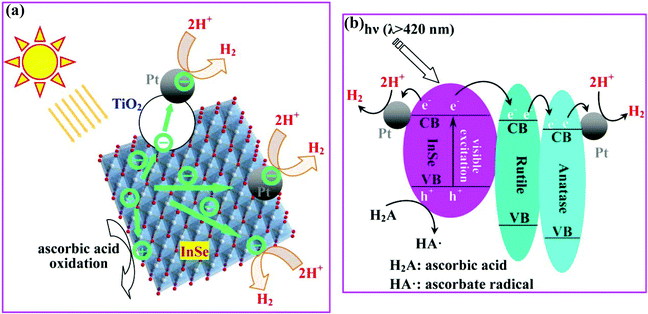
A schematic illustration of the charge separation and transfer in the InSe–TiO2 heterostructure is shown in Fig. 4. Driven by visible light, electrons (e−) are excited from the VB to the CB of the InSe semiconductor and then likely transfer in three ways (Fig. 4a): (1) to the active sites on the InSe nanosheets; (2) to Pt deposited on the surface of InSe nanosheets; (3) to Pt located on the TiO2 nanoparticles. In our study, there exists intimate contact among InSe, rutile and anatase in the InSe–TiO2 heterostructure. The interface among the three phases can act as a rapid separation site for the photogenerated electrons and holes due to the difference in the energy levels of their conduction bands (CB) and valence bands (VB). As shown in Fig. 4b, the heterojunction and phase junction formed between InSe and rutile, and rutile and anatase favor the transfer of photoexcited electrons from InSe to rutile, and then to anatase, thereby effectively suppressing the recombination of photoexcited electrons and holes.
In summary, a novel SDBS-assisted two-step method was used to prepare unusual cubic phase InSe nanosheets. The resulting InSe nanosheets were firstly used as catalysts for H2 production under visible light irradiation. More importantly, P25 was introduced into these new photocatalysts and highly efficient InSe–TiO2 (anatase and rutile) photocatalysts were produced. It could be found that the InSe–TiO2(0.015) heterostructure exhibits the highest H2 generation rate, which was found to increase by 4.1 times compared to that of pure InSe. The enhanced activity was mainly attributed to the interfacial transfer of photogenerated electrons from InSe to rutile, and then to anatase, leading to the effective charge separation and transfer on this semiconductor, which were evidenced by HRTEM, contact angle and photocurrent analysis. Under the optimized conditions, the apparent quantum efficiency was 25.5% at 450 nm with 2.5 wt% Pt as a cocatalyst. These findings may offer a promising route to obtain high performance nanohybrids for photocatalytic applications.
Acknowledgements
This work was supported by the Natural Science Foundation of China (NSFC, no. 21173021, 21231002, 21276026, 21471016 and 21271023), 973 Program (2014CB932103), and the 111 Project (B07012).Notes and references
- H. B. Gray, Nat. Chem., 2009, 1, 112 CrossRef CAS PubMed.
- X. Wu, S. Yin, Q. Dong and T. Sato, Appl. Catal., B, 2014, 156–157, 257 CrossRef CAS PubMed.
- (a) H. Yin and Z. Tang, ChemCatChem, 2015, 7, 904 CrossRef CAS PubMed; (b) X. Ma, K. Zhao, H. Tang, Y. Chen, C. Lu, W. Liu, Y. Gao, H. Zhao and Z. Tang, Small, 2014, 10, 4664 CrossRef CAS PubMed; (c) H. Yin, S. Zhao, K. Zhao, A. Muqsit, H. Tang, L. Chang, H. Zhao, Y. Gao and Z. Tang, Nat. Commun., 2015, 6, 6430 CrossRef PubMed.
- (a) F. A. Frame and F. E. Osterloh, J. Phys. Chem. C, 2010, 114, 10628 CrossRef CAS; (b) P. Tongying, V. V. Plashnitsa, N. Petchsang, F. Vietmeyer, G. J. Ferraudi, G. Krylova and M. Kuno, J. Phys. Chem. Lett., 2012, 3, 3234 CrossRef CAS; (c) J. U. Bang, S. J. Lee, J. S. Jang, W. Choi and H. Song, J. Phys. Chem. Lett., 2012, 3, 3781 CrossRef CAS; (d) M. A. Holmes, T. K. Townsend and F. E. Osterloh, Chem. Commun., 2012, 48, 371 RSC; (e) Y. Peng, L. Shang, T. Bian, Y. Zhao, C. Zhou, H. Yu, L. Wu, C. Tung and T. Zhang, Chem. Commun., 2015, 51, 4677 RSC.
- F. A. Frame, E. C. Carroll, D. S. Larsen, M. Sarahan, N. D. Browning and F. E. Osterloh, Chem. Commun., 2008, 2206 RSC.
- (a) W. Zhang and R. Xu, Int. J. Hydrogen Energy, 2012, 37, 17899 CrossRef CAS PubMed; (b) D. Wei, Z. Lin, Z. Cui, S. Su, D. Zhang, M. Cao and C. Hu, Chem. Commun., 2013, 49, 9609 RSC.
- (a) W. Feng, X. Zhou, W. Q. Tian, W. Zheng and P. A. Hu, Phys. Chem. Chem. Phys., 2015, 17, 3653 RSC; (b) S. R. Tamalampudi, Y. Y. Lu, U. R. Kumar, R. Sankar, C. D. Liao, B. K. Moorthy, C. H. Cheng, F. C. Chou and Y. T. Chen, Nano Lett., 2014, 14, 2800 CrossRef CAS PubMed; (c) B. Kobbi and N. Kesri, Vacuum, 2004, 75, 177 CrossRef CAS PubMed.
- M. Parlak and C. Ercelebi, Thin Solid Films, 1998, 322, 334 CrossRef CAS.
- (a) W. J. Li, Y. N. Zhou and Z. W. Fu, Appl. Surf. Sci., 2011, 257, 2881 CrossRef CAS PubMed; (b) M. L. Madugu, L. Bowen, O. K. Echendu and I. M. Dharmadasa, J. Mater. Sci.: Mater. Electron., 2014, 25, 3977 CrossRef CAS.
- K. H. Park, K. Jang, S. Kim, H. J. Kim and S. U. Son, J. Am. Chem. Soc., 2006, 128, 14780 CrossRef CAS PubMed.
- (a) J. Li, J. Zhang and Y. T. Qian, Solid State Sci., 2008, 10, 1549 CrossRef CAS PubMed; (b) Z. Liu, Y. Yang, J. Liang, Z. Hu, S. Li, S. Peng and Y. T. Qian, J. Phys. Chem. B, 2003, 107, 12658 CrossRef CAS; (c) Z. Liu, S. Li, Y. Yang, S. Peng, Z. Hu and Y. T. Qian, Adv. Mater., 2003, 15, 1946 CrossRef CAS PubMed; (d) Y. D. Li, C. W. Li, H. R. Wang, L. Q. Li and Y. T. Qian, Mater. Chem. Phys., 1999, 59, 88 CrossRef CAS; (e) J. Wan, X. Chen, Z. Wang, W. Yu and Y. T. Qian, Mater. Chem. Phys., 2004, 88, 217 CrossRef CAS PubMed; (f) H. Fan, M. Zhang, X. Zhang and Y. T. Qian, J. Cryst. Growth, 2009, 311, 4530 CrossRef CAS PubMed.
- J. Wang, X. Yang, K. Zhao, P. Xu, L. Zong, R. Yu, D. Wang, J. Deng, J. Chen and X. Xing, J. Mater. Chem. A, 2013, 1, 9069 CAS.
- C. Hsin, W. Lee, C. T. Huang, C. W. Huang, W. Wu and L. Chen, Nano Lett., 2011, 11, 4348 CrossRef CAS PubMed.
- X. Pian, B. Lin, Y. Chen, J. Kuang, K. Zhang and L. Fu, J. Phys. Chem. C, 2011, 115, 6531 CAS.
- (a) Z. Han, F. Qiu, R. Eisenberg, P. L. Holland and T. D. Krauss, Science, 2012, 338, 1321 CrossRef CAS PubMed; (b) J. Ran, J. Yu and M. Jaroniec, Green Chem., 2011, 13, 2708 RSC; (c) S. Lei, J. Yu, S. Bao, G. Zeng, H. Liu, D. Wu, X. Tang, J. Zou and C. Au, Appl. Catal., A, 2015, 493, 58 CrossRef CAS PubMed; (d) H. Wang, W. Wang, G. Si, F. Wang, C. Tung and L. Wu, Langmuir, 2010, 26, 9766 CrossRef CAS PubMed.
- (a) M. Khan, S. Woo and O. Yang, Int. J. Hydrogen Energy, 2008, 33, 5345 CrossRef CAS PubMed; (b) Y. Ou, J. Lin, S. Fang and D. Liao, Chem. Phys. Lett., 2006, 429, 199 CrossRef CAS PubMed.
- (a) H. Tang, C. M. Hessel, J. Wang, N. Yang, R. Yu, H. Zhao and D. Wang, Chem. Soc. Rev., 2014, 43, 4281 RSC; (b) J. Du, X. Lai, N. Yang, J. Zhai, D. Kisailus, F. Su, D. Wang and L. Jiang, ACS Nano, 2011, 5, 590 CrossRef CAS PubMed.
- S. Chen, S. Shen, G. Liu, Y. Qi, F. Zhang and C. Li, Angew. Chem., Int. Ed., 2015, 54, 3047 CrossRef CAS PubMed.
- (a) J. Y. Yu, S. D. Zhuang, X. Y. Xu, W. C. Zhu, B. Feng and J. G. Hu, J. Mater. Chem. A, 2015, 3, 1199 RSC; (b) F. Cai, Y. Tang, H. Shen, C. Wang, A. Ren, L. Xiao, W. Gu and W. Shi, CrystEngComm, 2015, 17, 1086 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: The experimental details, results of SEM and photocatalytic H2 production rates. See DOI: 10.1039/c5qi00075k |
| This journal is © the Partner Organisations 2015 |