Open metal sites dangled on cobalt trigonal prismatic clusters within porous MOF for CO2 capture†
Wen-Yang
Gao
a,
Sathvik
Palakurty
a,
Lukasz
Wojtas
a,
Yu-Sheng
Chen
b and
Shengqian
Ma
*a
aDepartment of Chemistry, University of South Florida, 4202 E. Flower Avenue, Tampa, Florida 33620, USA. E-mail: sqma@usf.edu; Fax: +1-813-974-3203; Tel: +1-813-974-5217
bChemMatCARS, Center for Advanced Radiation Sources, The University of Chicago, 9700 S. Cass Avenue, Argonne, Illinois 60439, USA
First published on 26th January 2015
Abstract
Open metal sites are devised to dangle on cobalt trigonal prismatic secondary building units (SBUs) of a porous MOF, when a rigid octacarboxylate ligand, 1,3,6,8-tetra(3,5-dicarboxylphenyl)pyrene, assembled with Co(NO3)2 under solvothermal conditions. The functional role of dangled open metal sites is exploited for CO2 capture studies.
Over the last two decades, metal–organic frameworks (MOFs)1 have been established as a promising type of porous material that have revolutionized the fields of materials science and adsorbent development since their discovery. Built from metal ions or in situ generated clusters (also known as secondary building units (SBUs)) and organic ligands, MOFs have been bestowed with the distinctive features of designability and modularity, which means that a desired framework can thus be targeted by judicious selection of the SBU and organic ligand.2 Furthermore, the modularity of MOFs implies that their properties (for example, pore size, pore walls and surface area) can also be fine-tuned by custom design of organic ligands on the basis of certain SBUs.3 Their features such as designability of structures, and tunability of properties by virtue of crystal engineering strategies4 endow MOFs with great potential for practical applications in a variety of areas, such as gas adsorption,5 gas separation,6 heterocatalysis,7 sensors8 and others.9
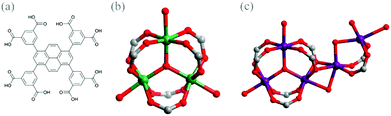
Amongst more than 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 structures studied, a large number of them remains, however sustained by only a handful of highly symmetrical SBUs, predominated by octahedral [Zn4O(COO)6], square paddlewheel [Cu2(COO)4] and trigonal prismatic [M3(μ3-O)(COO)6] (M = Cr, Fe, Mn, Ni, In and others) clusters.2,10 Given that the [M3(μ3-O)(COO)6] SBU (Fig. 1(b)) demonstrates remarkable robustness and stability, the trigonal prismatic cluster facilitates a plethora of prototype polyhedral nets and highly porous materials, as exemplified by MIL-100 and MIL-101.11 However, according to CSD search, there are very limited porous MOF structures by virtue of the [Co3(μ3-O)(COO)6] cluster as the SBU to build MOF networks.12 In this contribution, we report a unique example of a porous MOF (labelled as 1) assembled from [Co3(μ3-O)(COO)6]. The extra open metal sites are observed for the first time to be dangled on the cobalt trigonal prismatic clusters, which provide active interaction towards guest molecules and other species. CO2 adsorption studies are thus exploited on these interesting porous MOFs, featuring dangled open metal sites.
000 structures studied, a large number of them remains, however sustained by only a handful of highly symmetrical SBUs, predominated by octahedral [Zn4O(COO)6], square paddlewheel [Cu2(COO)4] and trigonal prismatic [M3(μ3-O)(COO)6] (M = Cr, Fe, Mn, Ni, In and others) clusters.2,10 Given that the [M3(μ3-O)(COO)6] SBU (Fig. 1(b)) demonstrates remarkable robustness and stability, the trigonal prismatic cluster facilitates a plethora of prototype polyhedral nets and highly porous materials, as exemplified by MIL-100 and MIL-101.11 However, according to CSD search, there are very limited porous MOF structures by virtue of the [Co3(μ3-O)(COO)6] cluster as the SBU to build MOF networks.12 In this contribution, we report a unique example of a porous MOF (labelled as 1) assembled from [Co3(μ3-O)(COO)6]. The extra open metal sites are observed for the first time to be dangled on the cobalt trigonal prismatic clusters, which provide active interaction towards guest molecules and other species. CO2 adsorption studies are thus exploited on these interesting porous MOFs, featuring dangled open metal sites.
Purple block-shaped crystals of 1 were obtained by reacting Co(NO3)2·6H2O with the 1,3,6,8-tetra(3,5-dicarboxyphenyl)pyrene (H8tdcppy) ligand (Fig. 1(a))13 in a solvent mixture of N,N-dimethylformamide (DMF)–methanol–H2O under solvothermal conditions for 36 hours (see ESI†). Single-crystal X-ray diffraction analysis conducted at the Advanced Photo Source, Argonne National Laboratory revealed that 1 crystallizes in the space group of Pnma.‡ Close inspection of the structure of 1 indicates that an exceptional type of trigonal prismatic cluster is employed as the mono-SBU to merge into a three-dimensional (3D) framework. A conventional trigonal prismatic SBU is composed of three octahedrally coordinated metal ions linked to a central oxygen ion in a planar environment, serving as 3-, 6-, 9-connected nodes. It is worth mentioning here that three open metal sites of the trigonal prism of 1 are occupied by disordered solvent molecules, providing oxygen atoms as binding sites. Exceptionally, in 1, two partially occupied cobalt sites are dangled around the trigonal prismatic cluster (Fig. 1(c)), which consists of trimeric cobalt cations interconnected by six carboxylate groups from six different tdcppy ligands and bridged by a μ3-O in the centre. The neighbouring Co4 with 0.61 occupancy coordinates to six oxygen atoms, two of which are ligated by two μ2-O from the carboxylate group of the trigonal prism, two from another two independent carboxylate groups of two different tdcppy ligands, and two from the μ2-O of the disordered bridging solvents. The other adjacent Co site (split into Co5 (occupancy = 0.20) and Co6 (occupancy = 0.20)) is not only bridged by the two independent carboxylate groups with Co4, but also linked by a μ2-O of the disordered bridging water with Co4. In addition, the corresponding fourth and fifth coordinated oxygen atoms to this Co site come from the solvent molecules. While eight carboxylate groups coordinatively bind to each of the cobalt multinuclear cluster (distorted cobalt trigonal prism), they are provided by only six individual tdcppy ligands. In terms of the octacarboxylate ligand of tdcppy, six carboxylate groups participate in composing the regular parts of six trigonal prismatic clusters and two carboxylate groups functionalize in bridging the dangled cobalt sites.
Topologically, 1 can be described as a binodal nia network derived from TOPOS analysis,14 in which each distorted cobalt trigonal prismatic SBU, serving as a 6-connected trigonal prismatic node, is bridged by six tdcppy ligands serving as the 6-connected octahedral nodes. As demonstrated, combining the rather robust trigonal prismatic cluster with the rigid tdcppy ligand, the framework of 1 is generated to be a porous 3D structure (shown in Fig. 2(a) and (b)) with certain expected robustness. Meanwhile, the combination of the default robust SBU and the rigid ligand compromises with each other, ending up with the “flexible” adapted or distorted metal clusters, as further exemplified by the previously reported MMPF-2.15
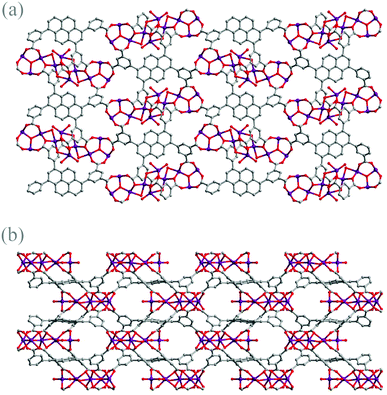 | ||
| Fig. 2 (a) The structure of 1 viewed along the b direction and (b) the structure of 1 viewed along the c direction. | ||
The phase purity of 1 was verified by powder X-ray diffraction (PXRD) studies, which indicate that the diffraction patterns of the fresh sample are consistent with the calculated ones (Fig. S1, ESI†). Thermogravimetric analysis (TGA) was performed on the fresh sample of 1 (Fig. S2, ESI†). A continuous weight loss of ∼34% from 25 °C to ∼90 °C is observed, corresponding to the loss of guest solvent molecules trapped in the cavities of 1. The plot is followed by a relatively steady plateau from 90 °C to 190 °C. Before its decomposition at ∼420 °C, the TGA plot shows a two-step slow weight loss from ∼61% to ∼43%, which corresponds with the monodentate and bidentate guest solvent molecules, respectively. TGA studies were also conducted on the activated sample of 1. It reveals that a continuous weight loss of ∼15% from 25 °C to 120 °C, which originates from the absorbed moisture, while the activated sample is exposed to the air prior to the TGA test. Then the plot is followed by a constant plateau until 420 °C before complete collapse of the framework. This result underlines the high affinity of the dangled open metal sites to the moisture, and highlights the robustness of the structure of 1. Furthermore, this also confirms that the activation has removed guest species or coordinated solvent molecules from the sample of 1.
To examine the permanent porosity of 1, gas adsorption studies were performed on the above-mentioned activated sample. As shown in Fig. 3(a), the N2 adsorption isotherm collected at 77 K indicates that 1 exhibits an uptake capacity of ∼147 cm3 g−1 at 1 atmospheric pressure with typical type-I adsorption behaviour of microporous materials. On the basis of the N2 adsorption data at 77 K, 1 possesses a Brunauer–Emmett–Teller (BET) surface area of ∼554 m2 g−1 (P/P0 = 0.0001–0.1), corresponding to a Langmuir surface area of ∼625 m2 g−1 (P/P0 = 0.0001–0.1). Density functional theory (DFT) pore size distribution analysis (Fig. S3, ESI†) based on the N2 adsorption data at 77 K reveals that the pore size of 1 is narrowly distributed at around 8.0 Å and 11.0 Å, which is in good agreement with the width of the channels observed in the crystal structure from b and c axes, respectively.
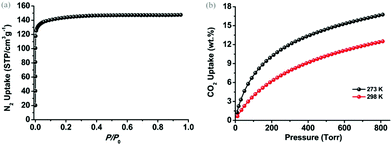 | ||
| Fig. 3 (a) N2 adsorption isotherm of 1 at 77 K and (b) CO2 adsorption isotherms of 1 at 273 K (black) and 298 K (red). | ||
It has been well-documented that open metal sites (unsaturated metal centers) in porous MOFs play a crucial role in increasing gas binding affinity, facilitating gas selectivity and creating catalytically active centers.16 However, the dangled metal sites in this case, as a peculiar type of open metal site, are rarely discussed and explored. Thus these dangled open metal sites on the trigonal prism prompted us to exploit their functional role in CO2 uptake performances, which provided the high affinity of the activated sample to the moisture. As shown in Fig. 2(b), 1 can adsorb substantial amounts of CO2 with the uptake capacities of 16.4 wt% (83.5 cm3 g−1) at 273 K and 12.1 wt% (61.6 cm3 g−1) at 298 K under 1 atmospheric pressure. Considering the practical conditions of atmospheric pressure and room temperature, 1 exhibits a comparable uptake value to the prototype NH2-MIL-53(Al) that features highly active amine groups (12.0 wt%, 298 K, 1 atm)17 but is moderate compared to some highly porous MOFs6a,18 due to the low surface area of 1. However, the shape of CO2 adsorption isotherms manifests the relatively strong interactions between the framework of 1 and CO2 gas molecules.19 The heats of adsorption (Qst) of CO2 for 1 were calculated based on CO2 adsorption isotherms at 273 K and 298 K using the virial method. As shown in Fig. S4, ESI,† the Qst of 1 is steadily yet slowly dropped from the initial value of ∼30 kJ mol−1 to ∼27 kJ mol−1 at high loadings, which distinguishes itself from other MOFs decorated with primary amine groups or open metal sites, whose Qst usually decreases abruptly to 20–22 kJ mol−1 with the increase of CO2 loading.20 This can be tentatively attributed to the high density of open metal sites allocated on the limited accessible surface of 1.
In summary, a porous MOF, 1, has been assembled from the cobalt-based trigonal prismatic SBU and the rigid pyrene-derived octacarboxylate ligand, which features open metal sites dangled on the trigonal prism. 1 represents the first porous MOF constructed by cobalt-trigonal prismatic clusters. 1 exhibits permanent porosity with a Langmuir surface area of ∼625 m2 g−1, and demonstrates high affinity to CO2 gas molecules. The strategy of employing a rigid organic ligand with a default robust SBU opens up a tentative way to build some “flexible” adapted or distorted SBU.
The authors acknowledge the University of South Florida and the National Science Foundation (DMR-1352065) for financial support of this work. We thank Prof. Randy W. Larsen and Christi L. Whittington for assistance with fluorescence tests and helpful discussions. The single-crystal X-ray diffraction of 1 was carried out at the Advanced Photon Source ChemMatCARS Sector 15, supported by the National Science Foundation/Department of Energy under grant number NSF/CHE-1346572. Use of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the U.S. DOE under Contract no. DE-AC02-06CH11357.
Notes and references
- (a) H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673 CrossRef CAS PubMed; (b) H.-C. Zhou and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5415 RSC.
- (a) S. Qiu and G. Zhu, Coord. Chem. Rev., 2009, 253, 2891 CrossRef CAS PubMed; (b) W. Lu, Z. Wei, Z.-Y. Gu, T.-F. Liu, J. Park, J. Park, J. Tian, M. Zhang, Q. Zhang, T. Gentle III, M. Bosch and H.-C. Zhou, Chem. Soc. Rev., 2014, 43, 5561 RSC.
- (a) J. J. Perry, J. A. Perman and M. J. Zaworotko, Chem. Soc. Rev., 2009, 38, 1400 RSC; (b) H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed; (c) W.-Y. Gao and S. Ma, Comments Inorg. Chem., 2014, 34, 125 CrossRef CAS.
- (a) G. R. Desiraju, Crystal Engineering: The Design of Organic Solids, Elsevier, Amsterdam, 1989 Search PubMed; (b) B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629 CrossRef CAS PubMed.
- (a) S. Ma and H.-C. Zhou, Chem. Commun., 2010, 46, 44 RSC; (b) H. Wu, Q. Gong, D. H. Olson and J. Li, Chem. Rev., 2012, 112, 836 CrossRef CAS PubMed; (c) Y. He, W. Zhou, G. Qian and B. Chen, Chem. Soc. Rev., 2014, 43, 5657 RSC.
- (a) K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724 CrossRef CAS PubMed; (b) J.-R. Li, J. Sculley and H.-C. Zhou, Chem. Rev., 2012, 112, 869 CrossRef CAS PubMed; (c) E. Barea, C. Montoro and J. A. R. Navarro, Chem. Soc. Rev., 2014, 43, 5419 RSC.
- (a) M. Yoon, R. Srirambalaji and K. Kim, Chem. Rev., 2012, 112, 1196 CrossRef CAS PubMed; (b) A. Dhakshinamoorthy and H. Garcia, Chem. Soc. Rev., 2014, 43, 5750 RSC; (c) J. Liu, L. Chen, H. Cui, J. Zhang, L. Zhang and C.-Y. Su, Chem. Soc. Rev., 2014, 43, 6011 RSC; (d) T. Zhang and W. Lin, Chem. Soc. Rev., 2014, 43, 5982 RSC.
- (a) L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105 CrossRef CAS PubMed; (b) Y. Cui, Y. Yue, G. Qian and B. Chen, Chem. Rev., 2012, 112, 1126 CrossRef CAS PubMed; (c) Z. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815 RSC.
- (a) W.-Y. Gao, M. Chrzanowski and S. Ma, Chem. Soc. Rev., 2014, 43, 5841 RSC; (b) V. Stavila, A. A. Talin and M. D. Allendorf, Chem. Soc. Rev., 2014, 43, 5994 RSC; (c) P. Ramaswamy, N. E. Wong and G. K. H. Shimizu, Chem. Soc. Rev., 2014, 43, 5913 RSC; (d) J.-P. Zhang, P.-Q. Liao, H.-L. Zhou, R.-B. Lin and X.-M. Chen, Chem. Soc. Rev., 2014, 43, 5789 RSC.
- A. Schoedel and M. J. Zaworotko, Chem. Sci., 2014, 5, 1269 RSC.
- (a) G. Férey, C. Serre, C. Mellot-Draznieks, F. Millange, S. Surblé, J. Dutour and I. Margiolaki, Angew. Chem., Int. Ed., 2004, 43, 6296 CrossRef PubMed; (b) G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé and I. Margiolaki, Science, 2005, 309, 2040 CrossRef PubMed.
- (a) F. H. Allen, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 380 CrossRef PubMed; (b) X.-M. Zhang, Y.-Z. Zheng, C.-R. Li, W.-X. Zhang and X.-M. Chen, Cryst. Growth Des., 2007, 7, 980 CrossRef CAS; (c) C.-S. Lim, J. K. Schnobrich, A. G. Wong-Foy and A. J. Matzger, Inorg. Chem., 2010, 49, 5271 CrossRef CAS PubMed.
- N. Zhao, F. Sun, H. He, J. Jia and G. Zhu, Cryst. Growth Des., 2014, 14, 1738 CAS.
- V. A. Blatov, A. P. Shevchenko and D. M. Proserpio, Cryst. Growth Des., 2014, 14, 3576 CAS.
- X.-S. Wang, M. Chrzanowski, C. Kim, W.-Y. Gao, L. Wojtas, Y.-S. Chen, X. P. Zhang and S. Ma, Chem. Commun., 2012, 48, 7173 RSC.
- (a) B. Chen, N. W. Ockwig, A. R. Millward, D. S. Contreras and O. M. Yaghi, Angew. Chem., Int. Ed., 2005, 44, 4745 CrossRef CAS PubMed; (b) D. Britt, H. Furukawa, B. Wang, T. G. Glover and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 20637 CrossRef CAS PubMed; (c) E. D. Bloch, W. L. Queen, R. Krishna, J. M. Zadrozny, C. M. Brown and J. R. Long, Science, 2012, 335, 1606 CrossRef CAS PubMed; (d) W.-Y. Gao, Y. Chen, Y. Niu, K. Williams, L. Cash, P. J. Perez, L. Wojtas, J. Cai, Y.-S. Chen and S. Ma, Angew. Chem., Int. Ed., 2014, 53, 2615 CrossRef CAS PubMed.
- B. Arstad, H. Fjellvåg, K. O. Kongshaug, O. Swang and R. Blom, Adsorption, 2008, 14, 755 CrossRef CAS.
- J.-R. Li, Y. Ma, M. C. McCarthy, J. Sculley, J. Yu, H.-K. Jeong, P. B. Balbuena and H.-C. Zhou, Coord. Chem. Rev., 2011, 255, 1791 CrossRef CAS PubMed.
- W.-Y. Gao, Y. Niu, Y. Chen, L. Wojtas, J. Cai, Y.-S. Chen and S. Ma, CrystEngComm, 2012, 14, 6115 RSC.
- (a) A. Demessence, D. M. D'Alessandro, M. L. Foo and J. R. Long, J. Am. Chem. Soc., 2009, 131, 8784 CrossRef CAS PubMed; (b) T. M. McDonald, D. M. D'Alessandro, R. Krishna and J. R. Long, Chem. Sci., 2011, 2, 2022 RSC; (c) J. An, S. J. Geib and N. Rosi, J. Am. Chem. Soc., 2010, 132, 38 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, powder X-ray diffraction patterns, TGA plots, heats of adsorption, and single-crystal X-ray diffraction data. CCDC 1031895. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4qi00240g |
| ‡ X-ray crystal data for 1: C56.15H18Co4N2.65O24.68, fw = 1360.22, orthorhombic, Pnma, a = 33.152(2) Å, b = 14.4302(10) Å, c = 14.0799(10) Å, Z = 4, T = 100(2) K, ρcalcd = 1.341 g cm−3, R1(I > 2σ(I)) = 0.0705, wR2 (all data) = 0.2175, CCDC 1031895. |
| This journal is © the Partner Organisations 2015 |