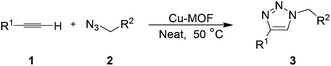
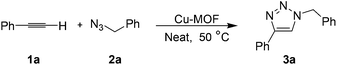
A metal–organic framework as a highly efficient and reusable catalyst for the solvent-free 1,3-dipolar cycloaddition of organic azides to alkynes†
Peng
Li
,
Sridhar
Regati
,
Huicai
Huang
,
Hadi D.
Arman
,
John C.-G.
Zhao
* and
Banglin
Chen
*
Department of Chemistry, University of Texas at San Antonio, One UTSA Circle, San Antonio, TX 78249-0698, USA. E-mail: banglin.chen@utsa.edu; cong.zhao@utsa.edu
First published on 14th November 2014
Abstract
A new highly efficient and reusable Cu-MOF has been developed for the regioselective synthesis of 1,2,3-triazoles via the 1,3-dipolar cycloaddition of organic azides to terminal alkynes under solvent-free conditions. This protocol has the advantages of excellent product yields and a low catalyst loading. Moreover, the catalyst may be recovered and reused efficiently up to 5 cycles without major loss of reactivity.
1,2,3-Triazoles are ubiquitous structural motifs found in a wide range of biologically active natural products and have various applications in pharmaceuticals, agrochemicals, biochemicals and materials science.1 Huisgen 1,3-dipolar cycloaddition of organic azides to terminal alkynes is one of the most important synthetic routes to triazole derivatives. The traditional Huisgen uncatalyzed thermal approaches results in the formation of a 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 1,4- and 1,5-regioisomers.2 In 2002, the groups of Sharpless3 and Meldal4 have independently discovered the highly regioselective Huisgen 1,3-dipolar cycloaddition of azides and alkynes catalyzed by Cu(I) species under mild conditions to afford 1,4-disubstituted 1,2,3-triazoles. This copper(I)-catalyzed azide–alkyne-cycloaddition (CuAAC) is now also known as the click reaction, which has found a myriad of applications in modern synthesis.5
1 mixture of 1,4- and 1,5-regioisomers.2 In 2002, the groups of Sharpless3 and Meldal4 have independently discovered the highly regioselective Huisgen 1,3-dipolar cycloaddition of azides and alkynes catalyzed by Cu(I) species under mild conditions to afford 1,4-disubstituted 1,2,3-triazoles. This copper(I)-catalyzed azide–alkyne-cycloaddition (CuAAC) is now also known as the click reaction, which has found a myriad of applications in modern synthesis.5
It is well known that a Cu(I) species is required for the catalyzed Huisgen 1,3-dipolar cycloadditions.5–7 The Cu(I) species are generally prepared by the in situ reduction of Cu(II) salts, such as using CuSO4 and sodium ascorbate as the precatalysts,6 by the in situ oxidation of Cu(0) metals,7 or by a Cu(II)/Cu(0) comproportionation.8 Later, it was found that Cu(I) species stabilized by various ligands such as nitrogen-9 and sulphur-containing compounds,10 N-heterocyclic carbenes (NHC),11 and polydentate ligands12 not only accelerate the catalytic process but also allow the direct use of Cu(I) salts as catalysts for this 1,3-dipolar cycloaddition. Most of the reported copper-catalyzed azide–alkyne cycloaddition reactions are homogeneous13 and have serious drawbacks such as the difficulty in separation and subsequent reuse of the expensive catalysts and the indispensability of additives such as stabilizing ligands and bases.
The development of robust, easily recoverable, and recyclable heterogeneous catalysts can potentially solve these problems, which has received particular research interest in the recent years.14 Indeed, the versatility of the click chemistry has driven the development of a large number of protocols in which various recoverable and reusable catalysts were employed, such as Cu-zeolites,14a–c Cu on charcoal,14d,e polymer-supported copper catalyst,14f,g Cu(0)/Al2O3,14h Cu(II)-hydrotalcite,14i Cu(0) nanocluster,14j CuBr on graphene oxide/Fe3O4,14k Cu/SiO2,14l Cu on nanosilica triazine dendrimer,14m Cu on resin,14n and Cu2O nanocrystals.14o
Unlike immobilized metal complexes, metal–organic frameworks (MOFs) are easily synthesized under mild reaction conditions and the pore volumes and/or surface area in MOFs and their functionalized organic linkers may be rationally designed and altered by introducing suitable metal ions and organic linkers. Heterogeneous catalysis is one of the most important applications of MOFs, mainly because the coordinatively unsaturated metal centers in MOFs may exhibit increased activity for catalysis and MOFs can maintain sufficient structural integrity for liquid phase reactions.15 Nevertheless, despite the progress made in the MOF-catalyzed organic reactions,16 MOFs have been rarely used as catalysts in the click chemistry.17 Herein, we describe the synthesis, characterization of a new Cu-MOF and its application for the azide–alkyne cycloaddition reaction under solvent-free conditions.
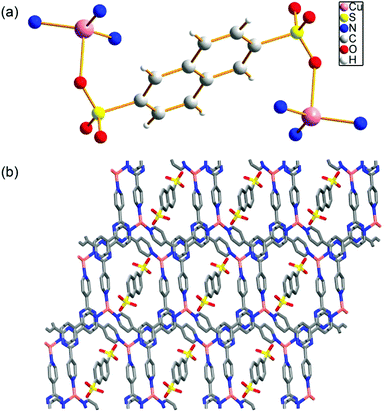
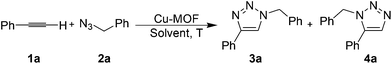
The Cu-MOF was readily synthesized from hydrothermal reactions from copper acetate, organic linkers 2,4,6-tri(4-pyridyl)-1,3,5-triazine (PTZ) and sodium 2,6-naphthalene disulfonate (NSA) in high yield (see the ESI†). Single-crystal structure of Cu-MOF indicates that it can be formulated as Cu(PTZ)(NSA)0.5·H2O. The purity of the phase was confirmed by powder XRD (Fig. S1†). The thermo gravimetric analysis (TGA) shows a clear loss of lattice water (obsd: 3.30 wt%; calcd: 3.36 wt%) from 100 °C to 170 °C and the de-solvated Cu-MOF can be stable up to 440 °C before decomposition (Fig. S2†). As shown in the crystallographic structure in Fig. 1a, each Cu ion is coordinated by three nitrogen atoms from three PTZ ligands and one oxygen atom from a sulfonate ligand in a tetrahedral coordination geometry. The ratio between the Cu ions and sulfonate ligands is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The oxidation state of Cu in the MOF was confirmed by X-ray photoelectron spectroscopy (Fig. S3†). The peak of binding energy of Cu2p at 932.50 eV, which was corrected with reference to C1 s (284.6 eV), is in good agreement with the data observed in Cu2O, indicating that Cu is +1.18 Due to the weak interactions between the sulfonate ligand and the Cu(I) ion [Cu–O distance is 2.370(2) Å], the Cu(I) site is readily accessible as a Lewis acid for potential heterogeneous catalysis. The packing diagram of Cu-MOF shows that the disulfonate serves as an anionic template bonding to the cationic layers (Fig. 1b).
1. The oxidation state of Cu in the MOF was confirmed by X-ray photoelectron spectroscopy (Fig. S3†). The peak of binding energy of Cu2p at 932.50 eV, which was corrected with reference to C1 s (284.6 eV), is in good agreement with the data observed in Cu2O, indicating that Cu is +1.18 Due to the weak interactions between the sulfonate ligand and the Cu(I) ion [Cu–O distance is 2.370(2) Å], the Cu(I) site is readily accessible as a Lewis acid for potential heterogeneous catalysis. The packing diagram of Cu-MOF shows that the disulfonate serves as an anionic template bonding to the cationic layers (Fig. 1b).
To test the catalytic activity of this novel MOF, we investigated the cycloaddition reaction between phenylacetylene (1a) and benzylazide (2a) using 5 mol% of Cu-MOF as the catalyst. The reaction was initially carried out at room temperature in CH2Cl2 as a solvent. As the results in Table 1 show, after 7 h, the desired 4-phenyltriazole (3a) was formed in an excellent yield (94%) and in a highly regioselective fashion (Table 1, entry 1). No formation of the regioisomer 5-phenyltrazole (4a) was observed in this reaction. The catalytic activity of the Cu-MOF in this reaction was unequivocally established by conducting a control experiment without the MOF catalyst: no formation of 3a was observed in the absence of the catalyst under otherwise identical reaction conditions (Table 1, entry 2). A screen of the catalyst loading (entries 3–6) revealed that the reaction proceeded well even with a catalyst loading of only 0.25 mol%, although the reaction took slightly longer time to complete (entry 6). A higher yield (92%) and shorter reaction time may be achieved at this loading by conducting the reaction under reflux conditions (entry 7). To our pleasure, the reaction also proceeded well with this low catalyst loading (0.25 mol%) under solvent-free conditions at rt (entry 8). At 50 °C, the reaction time was dramatically shortened to 2.5 h and a high yield of 3a (94%) was obtained (entry 9). Under these new conditions, we found that the reduction of the catalyst loading to 0.10 and 0.010 mol% resulted in longer reactions times and lower product yields (entries 10 and 11). Most importantly, these lower loadings led to the formation of a mixture of two regioisomeric products 3a and 4a, in ratios of 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 and 61
17 and 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39, respectively (entries 10 and 11). A control experiment under these conditions without the MOF catalyst showed that the lower regioselectivities observed were most likely due to the contribution of the background reaction, which in 24 h gave a mixture of products 3a and 4a in a ratio of 51
39, respectively (entries 10 and 11). A control experiment under these conditions without the MOF catalyst showed that the lower regioselectivities observed were most likely due to the contribution of the background reaction, which in 24 h gave a mixture of products 3a and 4a in a ratio of 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49, with yields of 40% and 39%, respectively (entry 12).
49, with yields of 40% and 39%, respectively (entry 12).
| Entry | Loading (mol%) | Solvent | Temp (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
a Unless otherwise indicated, all reactions were conducted with 1a (1.0 mmol), 2a (1.0 mmol) in the indicated solvent (1.0 mL).
b Yield of the isolated product. The number in the parentheses is the yield of the isolated 4a.
c The ratio of 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a was 83 4a was 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 according to the crude NMR.
d The ratio of 3a 17 according to the crude NMR.
d The ratio of 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a was 61 4a was 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 according to the crude NMR.
e The ratio of 3a 39 according to the crude NMR.
e The ratio of 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a was 51 4a was 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 according to the crude NMR. 49 according to the crude NMR.
|
|||||
| 1 | 5.0 | CH2Cl2 | rt | 7 | 94 |
| 2 | No catalyst | CH2Cl2 | rt | 7 | 0 |
| 3 | 2.5 | CH2Cl2 | rt | 7 | 93 |
| 4 | 1.0 | CH2Cl2 | rt | 14 | 92 |
| 5 | 0.5 | CH2Cl2 | rt | 24 | 92 |
| 6 | 0.25 | CH2Cl2 | rt | 32 | 87 |
| 7 | 0.25 | CH2Cl2 | Reflux | 12 | 92 |
| 8 | 0.25 | Neat | rt | 8 | 92 |
| 9 | 0.25 | Neat | 50 | 2.5 | 94 |
| 10 | 0.10 | Neat | 50 | 8 | 78 (12)c |
| 11 | 0.010 | Neat | 50 | 20 | 55 (34)d |
| 12 | No catalyst | Neat | 50 | 24 | 40 (39)e |
Once the reaction conditions were optimized, the scope of this cycloaddition reaction was investigated. The results are summarized in Table 2. As is evident from the results, besides phenylacetylene (entry 1), phenylacetylenes bearing electron-donating groups, such as 4-methyl (entry 2), 3-methyl (entry 3), or 4-methoxy (entry 4) groups, and electron-withdrawing groups, such as 4-bromo (entry 5), 2-bromo (entry 6), 3,5-difluoro (entry 7), and 2-trifluoromethyl (entry 8) groups, all underwent smooth reactions with benzylazide to yield the expected triazole 3 regioselectively in high yields (84–93%). 1-Ethynylnaphthalene also gave similar results to those of phenylacetylenes (entry 9). Alkyl-substituted acetylenes are also good substrates for this reaction. For example, phenylethyl-, 3-hydroxypropyl- and 1-hydroxycyclohexyl-substituted acetylenes all generated the desired products in high yields (entries 10–12). It is also worth noting that other functional groups, such as hydroxy (entries 11 and 12) and TMS (entry 13) groups, are tolerated in this reaction. In addition, the reaction of 4-methoxy-substituted benzylazide with phenylacetylene produced the expected product in 84% yield (entry 14). It needs to be pointed out that the Cu-MOF is a condensed structure, and there is no accessible channel, so the catalytic reactions take place on the surfaces of Cu-MOF. Because of this, the size effect of substrate does not determine the yield or reaction rate, while the electronic effect plays a major role, leading to the difference between entry 9 and 7.
| Entry | R1 | R2 | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a All reactions were conducted with acetylene 1 (1.0 mmol), benzylazide 2 (1.0 mmol), and Cu-MOF (0.25 mol%) at 50 °C for the indicated reaction times. b Yield of the isolated product. | ||||
| 1 | Ph | Ph | 2.5 | 94 |
| 2 | 4-MeC6H4 | Ph | 2.5 | 93 |
| 3 | 3-MeC6H4 | Ph | 2.8 | 92 |
| 4 | 4-MeOC6H4 | Ph | 2.5 | 92 |
| 5 | 4-BrC6H4 | Ph | 2.5 | 89 |
| 6 | 2-BrC6H4 | Ph | 2.5 | 87 |
| 7 | 3,5-F2C6H3 | Ph | 2 | 84 |
| 8 | 2-CF3C6H4 | Ph | 2.5 | 85 |
| 9 | 1-Naphthyl | Ph | 2 | 88 |
| 10 | PhC2H4 | Ph | 2.5 | 89 |
| 11 | OH(CH2)3 | Ph | 2.5 | 86 |
| 12 | 1-Hydroxycyclohexyl | Ph | 2.5 | 87 |
| 13 | TMS | Ph | 2.5 | 86 |
| 14 | Ph | 4-MeOC6H4 | 3 | 84 |
The main advantage of MOF catalysts lies in the fact that they can be easily recovered after the reaction due to their heterogeneity. Indeed, the Cu-MOF catalyst may be easily recovered using a centrifuge and washed with CH2Cl2 (5 mL) to remove traces of the previous reaction mixture and dried at 50 °C under vacuum for 2 h before using it for the next catalytic cycle. The PXRD pattern indicates the crystal structures of Cu-MOFs maintained after the catalytic reactions (Fig. S1†). Using 1a and 2a as the substrates, the reusability of the Cu-MOF catalyst was examined. As shown by the results in Table 3, the Cu-MOF catalyst may be recovered and reused for 5 cycles without any significant loss in catalytic activity. The main purpose of the recyclability study is to prove that Cu-MOF is still catalytically active after 5 recycles, though the time required in cycles 4 and 5 for complete conversion is slightly longer than those in the previous cycles.
| Entry | Reuse | Time (h) | Yieldb (%) |
|---|---|---|---|
| a All reactions were conducted with 1a, 2a, and Cu-MOF (0.25 mol%) at 50 °C for the indicated reaction times. b Yield of the isolated product 3a. c The reaction was carried out on a 3.0 mmol scale. d The reaction was carried out on a 2.0 mmol scale. e The reaction was carried out on a 1.0 mmol scale. f The reaction was carried out on a 0.50 mmol scale. g The reaction was carried out on a 0.40 mmol scale. | |||
| 1 | Cycle I | 2.5 | 94c |
| 2 | Cycle II | 2.5 | 92d |
| 3 | Cycle III | 3 | 90e |
| 4 | Cycle IV | 3 | 87f |
| 5 | Cycle V | 3.5 | 87g |
In conclusion, we have developed a new highly efficient Cu-MOF that catalyzes the high regioselective 1,3-dipolar cycloaddition of terminal alkynes to organic azides under solvent-free conditions. The desired triazoles were obtained in good to excellent yields in short reaction times. Many different functional groups are tolerated in this reaction. Moreover, the catalysts can be easily recovered and reused in this reaction without loss of the catalytic activity for at least five cycles.
Acknowledgements
J. Z. thanks the generous financial support for this research from the Welch Foundation (grant no. AX-1593) and the NIH-NIGMS (grant no. SC1GM082718); B.C. thanks the financial support for this research from the Welch Foundation (grant no. AX-1730).Notes and references
- (a) E.-H. Ryu and Y. Zhao, Org. Lett., 2005, 7, 1035 CrossRef CAS PubMed; (b) G. K. Such, J. F. Quinn, A. Quinn, E. Tjipto and F. Caruso, J. Am. Chem. Soc., 2006, 128, 9318 CrossRef CAS PubMed; (c) S. Lober, P. Rodriguez-Loaiza and P. Gmeiner, Org. Lett., 2003, 5, 1753 CrossRef PubMed; (d) J.-F. Lutz, Angew. Chem., Int. Ed., 2007, 46, 1018 CrossRef CAS PubMed; (e) R. Alvarez, S. Velazque, F. San, S. Aquaro, C. De, C. F. Perno, A. Karlsson, J. Balzarini and M. J. Camarasa, J. Med. Chem., 1994, 37, 4185 CrossRef CAS; (f) A. Brik, Y.-C. Lin, J. Elder and C.-H. Wong, Chem. Biol., 2002, 9, 891 CrossRef CAS.
- R. Huisgen, in 1,3-Dipolar Cycloaddition Chemistry, ed. A. Padwa, Wiley, New York, 1984, p. 1 Search PubMed.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS.
- C. Tornoe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef CAS PubMed.
- (a) S. K. Mamidyala and M. G. Finn, Chem. Soc. Rev., 2010, 39, 1252 RSC; (b) Y. Hua and A. H. Flood, Chem. Soc. Rev., 2010, 39, 1262 RSC; (c) C. Le Droumaguet, C. Wang and Q. Wang, Chem. Soc. Rev., 2010, 39, 1233 RSC; (d) K. D. Hanni and D. A. Leigh, Chem. Soc. Rev., 2010, 39, 1240 RSC; (e) J. E. Hein and V. V. Fokin, Chem. Soc. Rev., 2010, 39, 1302 RSC; (f) J. M. Holub and K. Kirshenbaum, Chem. Soc. Rev., 2010, 39, 1325 RSC; (g) M. Meldal and C. W. Tornøe, Chem. Rev., 2008, 108, 2952 CrossRef CAS PubMed; (h) V. D. Bock, H. Hiemstra and J. H. van Maarseveen, Eur. J. Org. Chem., 2006, 51 CrossRef CAS.
- K. B. Sharpless, V. V. Fokin, L. G. Green and V. V. Rostovtsev, Angew. Chem., Int. Ed., 2002, 114, 2708 CrossRef.
- F. Himo, T. Lovell, R. Hilgraf, V. V. Rostovtsev, L. Noodleman, K. B. Sharpless and V. V. Fokin, J. Am. Chem. Soc., 2005, 127, 210 CrossRef CAS PubMed.
- (a) Q. Wang, S. Chittaboina and H. N. Barnhill, Lett. Org. Chem., 2005, 2, 293 CrossRef CAS; (b) S. Quader, S. E. Boyd, I. D. Jenkins and T. A. Houston, J. Org. Chem., 2007, 72, 1962 CrossRef CAS PubMed.
- (a) V. O. Rodionov, S. I. Presolski, S. Gardinier, Y. H. Lim and M. G. Finn, J. Am. Chem. Soc., 2007, 129, 12696 CrossRef CAS PubMed; (b) V. O. Rodionov, S. I. Presolski, D. D. Diaz, V. V. Fokin and M. G. Finn, J. Am. Chem. Soc., 2007, 129, 12705 CrossRef CAS PubMed; (c) W. G. Lewis, F. G. Magallon, V. V. Fokin and M. G. Finn, J. Am. Chem. Soc., 2004, 126, 9152 CrossRef CAS PubMed; (d) T. R. Chan, R. Hilgraf, K. B. Sharpless and V. V. Fokin, Org. Lett., 2004, 6, 2853 CrossRef CAS PubMed.
- W. Wang, F. Hua, Y. Y. Jiang and Y. F. Zhao, Green Chem., 2008, 10, 452 RSC.
- (a) S. Diez-Gonzalez and S. P. Nolan, Angew. Chem., Int. Ed., 2008, 47, 8881 CrossRef CAS PubMed; (b) T. Nakamura, T. Terashima, K. Ogata and S-i. Fukuzawa, Org. Lett., 2011, 13, 620 CrossRef CAS PubMed.
- F. W. Li and T. S. A. Hor, Chem. – Eur. J., 2009, 15, 10585 CrossRef CAS PubMed.
- For selected examples of homogeneously catalyzed 1,3-dipolar cycloaddition reactions, see: (a) N. Candelon, D. Lastecoueres, A. K. Diallo, J. Ruiz Aranzaes, D. Astruc and J.-M. Vincent, Chem. Commun., 2008, 741 RSC; (b) S. Díez-González, A. Correa, L. Cavallo and S. P. Nolan, Chem. – Eur. J., 2006, 12, 7558 CrossRef PubMed; (c) L. Zhang, X. Chen, P. Xue, H. H. Y. Sun, I. D. Williams, K. B. Sharpless, V. V. Fokin and G. Jia, J. Am. Chem. Soc., 2005, 127, 15998 CrossRef CAS PubMed; (d) P. Appukkuttan, W. Dehaen, V. V. Fokin and E. van der Eycken, Org. Lett., 2004, 6, 4223 CrossRef CAS PubMed; (e) H. S. G. Beckmann and V. Wittmann, Org. Lett., 2007, 9, 1 CrossRef CAS PubMed; (f) B. Sreedhar and P. S. Reddy, Synth. Commun., 2007, 37, 805 CrossRef CAS; (g) B. Gerard, J. Ryan, A. B. Beeler and J. A. Porco, Jr., Tetrahedron, 2006, 62, 6405 CrossRef CAS PubMed; (h) Y.-B. Zhao, Z.-Y. Yan and Y.-M. Liang, Tetrahedron Lett., 2006, 47, 1545 CrossRef CAS PubMed; (i) B.-Y. Lee, S. R. Park, H. B. Jeon and K. S. Kim, Tetrahedron Lett., 2006, 47, 5105 CrossRef CAS PubMed; (j) K. Kamata, Y. Nakagawa, K. Yamaguchi and N. Mizuno, J. Am. Chem. Soc., 2008, 130, 15304 CrossRef CAS PubMed.
- For selected examples of heterogeneously catalyzed 1,3-dipolar cycloaddition reactions, see: (a) S. Chassaing, M. Kumarraja, A. S. S. Sido, P. Pale and J. Sommer, Org. Lett., 2007, 9, 883 CrossRef CAS PubMed; (b) S. Chassaing, A. S. S. Sido, A. Alix, M. Kumarraja, P. Pale and J. Sommer, Chem. – Eur. J., 2008, 14, 6713 CrossRef CAS PubMed; (c) V. Beneteau, A. Olmos, T. Boningari, J. Sommer and P. Pale, Tetrahedron Lett., 2010, 51, 3673 CrossRef CAS PubMed; (d) B. H. Lipshutz and B. R. Taft, Angew. Chem., Int. Ed., 2006, 45, 8235 CrossRef CAS PubMed; (e) H. Lopez-Ruiz, J. E. de la Cerda-Pedro, S. Rojas-Lima, I. Perez-Perez, B. V. Rodriguez-Sanchez, R. Santillan and O. Coreno, ARKIVOC, 2013, 139 CAS; (f) I. S. Park, M. S. Kwon, J. S. Lee and J. Park, Org. Lett., 2008, 10, 497 CrossRef CAS PubMed; (g) Y. Kitamura, K. Taniguchi, T. Maegawa, Y. Monguchi, Y. Kitade and H. Sajiki, Heterocycles, 2014, 88, 233 CrossRef CAS PubMed; (h) M. L. Kantam, V. S. Jaya, B. Sreedhar, M. M. Rao and B. M. Choudary, J. Mol. Catal. A: Chem., 2006, 256, 273 CrossRef CAS PubMed; (i) K. Namitharan, M. Kumarraja and K. Pitchumani, Chem. – Eur. J., 2009, 15, 2755 CrossRef CAS PubMed; (j) L. D. Pachn, J. H. van Maarseveen and G. Rothenberg, Adv. Synth. Catal., 2005, 347, 811 Search PubMed; (k) X. Q. Xiong, H. X. Chen, Z. K. Tang and Y. B. Jiang, RSC Adv., 2014, 4, 9830 RSC; (l) C. S. Radatz, L. D. Soares, E. R. Vieira, D. Alves, D. Russowsky and P. H. Schneider, New J. Chem., 2014, 38, 1410 RSC; (m) M. Nasr-Esfahani, I. Mohammadpoor-Baltork, A. R. Khosropour, M. Moghadam, V. Mirkhani, S. Tangestaninejad and H. A. Rudbari, J. Org. Chem., 2014, 79, 1437 CrossRef CAS PubMed; (n) Y. Monguchi, K. Nozaki, T. Maejima, Y. Shimoda, Y. Sawama, Y. Kitamura, Y. Kitade and H. Sajiki, Green Chem., 2013, 15, 490 RSC; (o) K. Chanda, S. Rej and M. H. Huang, Chem. – Eur. J., 2013, 19, 16036 CrossRef CAS PubMed.
- For reviews, see: (a) A. Dhakshinamoorthy and H. Garcia, Chem. Soc. Rev., 2014 10.1039/C1033CS60442J; (b) M. Yoon, R. Srirambalaji and K. Kim, Chem. Rev., 2012, 112, 1196 CrossRef CAS PubMed. For some selected examples, see: (c) H. H. Fei, D. L. Rogow and S. R. J. Oliver, J. Am. Chem. Soc., 2010, 132, 7202 CrossRef CAS PubMed; (d) Y. K. Hwang, D.-Y. Hong, J.-S. Chang, S. H. Jhung, Y.-K. Seo, J. Kim, A. Vimont, M. Daturi, C. Serre and G. Férey, Angew. Chem., Int. Ed., 2008, 47, 4144 CrossRef CAS PubMed; (e) H. Li, C. E. Davis, T. L. Groy, D. G. Kelley and O. M. Yaghi, J. Am. Chem. Soc., 1998, 120, 2186 CrossRef CAS; (f) P. M. Forster, J. Eckert, J.-S. Chang, S.-E. Park, G. Férey and A. K. Cheetham, J. Am. Chem. Soc., 2003, 125, 1309 CrossRef CAS PubMed; (g) P. Li, S. Regati, R. J. Butcher, H. D. Arman, Z. X. Chen, S. C. Xiang, B. L. Chen and C.-G. Zhao, Tetrahedron Lett., 2011, 52, 6220 CrossRef CAS PubMed; (h) M. Thimmaiah, P. Li, S. Regati, B. L. Chen and J. C.-G. Zhao, Tetrahedron Lett., 2012, 53, 4870 CrossRef CAS PubMed; (i) G. Lu, S. Li, Z. Guo, O. K. Farha, B. G. Hauser, X. Qi, Y. Wang, X. Wang, S. Han, X. Liu, J. S. DuChene, H. Zhang, Q. Zhang, X. Chen, J. Ma, S. C. J. Loo, W. D. Wei, Y. Yang, J. T. Hupp and F. Huo, Nat. Chem., 2012, 4, 310 CrossRef CAS PubMed; (j) W. Zhang, G. Lu, C. Cui, Y. Liu, S. Li, W. Yan, C. Xing, Y. R. Chi, Y. Yang and F. Huo, Adv. Mater., 2014, 26, 4056 CrossRef CAS PubMed; (k) M. Zhao, K. Deng, L. He, Y. Liu, G. Li, H. Zhao and Z. Tang, J. Am. Chem. Soc., 2014, 136, 1738 CrossRef CAS PubMed.
- (a) C. D. Wu and W. B. Lin, Angew. Chem., Int. Ed., 2007, 46, 1075 CrossRef CAS PubMed; (b) W. Y. Gao, Y. Chen, Y. H. Niu, K. Williams, L. Cash, P. J. Perez, L. Wojtas, J. F. Cai, Y. S. Chen and S. Q. Ma, Angew. Chem., Int. Ed., 2014, 53, 2615 CrossRef CAS PubMed; (c) Y. Liu, W. M. Xuan and Y. Cui, Adv. Mater., 2010, 22, 4112 CrossRef CAS PubMed.
- I. Luz, F. X. Llabrés i Xamena and A. Corma, J. Catal., 2010, 276, 134 CrossRef CAS PubMed.
- C. D. Wagner, W. M. Riggs, L. E. Davis, J. E. Moulder and G. E. Muilenber, Handbook of X-ray Photoelectron Spectroscopy, Perkin Elmer Corporation Physical Electronics Division, USA, 1979 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Crystal structure and PXRD of the Cu-MOF, detailed experimental procedures, and characterization data for a new product. CCDC 1013552. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4qi00148f |
| This journal is © the Partner Organisations 2015 |