 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Controlling the synthesis of degradable vinyl polymers by xanthate-mediated polymerization†
Craig A.
Bell
abc,
Guillaume G.
Hedir
a,
Rachel K.
O'Reilly
*a and
Andrew P.
Dove
*a
aDepartment of Chemistry, University of Warwick, Gibbet Hill Road, Coventry, CV4 7AL, UK. E-mail: r.k.o-reilly@warwick.ac.uk; a.p.dove@warwick.ac.uk
bAustralian Institute for Bioengineering and Nanotechnology, The University of Queensland, St Lucia, Queensland 4072, Australia
cCentre for Advanced Imaging, The University of Queensland, St Lucia, Queensland 4072, Australia
First published on 2nd September 2015
Abstract
The copolymerization of vinyl acetate (VAc) and 2-methylene-1,3-dioxepane (MDO), as well as the homopolymerization of MDO in the presence of a p-methoxyphenyl xanthate chain transfer agent (CTA) is reported and comparison of the homopolymerization of MDO with other known xanthates was also investigated. In depth investigation showed loss of the xanthate functionality was a result of Z-group fragmentation leading to the formation of carbonodithioate groups, as confirmed by 13C NMR spectroscopy. The use of the xanthate with a substituted phenyl Z-group drastically reduces fragmentation through the Z-group and hence significantly increases chain-end retention during the polymerization using the RAFT/MADIX technique. Post-polymerization modification of the chain-end of poly(MDO) was achieved by in situ aminolysis and base-catalyzed Michael addition of propargyl methacrylate onto the terminal thiol to form alkyne functional poly(MDO).
Introduction
Degradable polymers obtained by radical ring-opening polymerization (rROP) of cyclic ketene acetals (CKAs) have recently attracted significant interest as they represent a facile alternative to conventional ring-opening polymerization for the synthesis of aliphatic polyesters.1–3 The 5-, 6- and 7-membered CKAs formed from the appropriate aliphatic diols, as well as the methyl- and phenyl- substituted versions have been used to afford a range of polyester materials.4–6 The 7-membered CKA, 2-methylene-1,3-dioxepane (MDO), has been the most widely studied as a consequence of the polymer's repeat unit being identical to poly(ε-caprolactone) (PCL),7,8 a degradable polyester that is widely studied and applied in the biomedical field.9–11 Conventional methodologies to synthesize PCL typically use anionic or metal-catalyzed ROP of ε-caprolactone and require rigorous synthetic procedures in order to produce polymers of high purity.12,13 In contrast, poly(MDO) can be synthesized by conventional radical polymerization techniques and is therefore more easily accessible, requiring less stringent synthetic conditions.The mechanism for rROP involves the formation of a primary radical through electronic rearrangement and β-scission. As such, copolymers of MDO have been synthesized using a vast array of radically-polymerizable vinyl monomers. These include hydrophobic monomers such as ethylene,14 styrene (Sty),14–19 acrylonitrile (AN),19,20 vinyl acetate (VAc),21–23 and methyl methacrylate (MMA)20,24,25 as well as hydrophilic monomers such as poly(ethylene glycol) methyl ether methacrylate (PEGMA),26,27N,N-dimethylaminoethyl methacrylate (DMAEMA),28–30N-isopropylacrylamide (NIPAM),31,32 and N-vinylpyrrolidone (NVP).33,34 However, with the exception of less activated monomers (LAMs) such as VAc, the reactivity ratios for these copolymerizations indicate that final polymer compositions are more gradient-like or blocky, not statistical.17,25,29,35
Control of polymerizations incorporating CKAs has also been attempted through reversible-deactivation radical polymerization (RDRP) techniques such as Nitroxide-Mediated Polymerization (NMP),19,36 Atom Transfer Radical Polymerization (ATRP),37–39 and Reversible Addition-Fragmentation Chain-Transfer Polymerization/Macromolecular Design by Interchange of Xanthates (RAFT/MADIX)40 but there are only a handful of examples where these techniques have been used to control copolymerizations with MDO. Using BlocBuilder MA alkoxyamine initiator (SG1) to mediate the copolymerization of PEGMA or MMA, AN, and MDO, Delplace et al. have demonstrated control with final dispersities, ĐM, <1.4. However, the molar feeds used were only 20 or 40 mol%, and all MDO incorporations into the final polymers were determined qualitatively from hydrolytic degradation.19,20 We have also recently shown control over VAc/MDO copolymerizations. Using xanthates to mediate the polymerization, initial MDO monomer feeds of 30 and 70 mol% yielded polymers with predictable molecular weights and final dispersities, ĐM, <1.6. Additionally, these polymers were shown to have retained chain-end functionality through chain growth of VAc.22
Homopolymerization of MDO has predominantly been performed by free radical polymerization (FRP) and there are only a handful of studies that have attempted to synthesize poly(MDO) using RDRP techniques.41,42 The use of NMP enabled the controlled synthesis of polymers with greater degrees of control than conventional FRP. However, these studies enlisted the use of TEMPO as the mediating nitroxide, thus requiring high reaction temperatures and long polymerization times to obtain Mn ≤ 8.5 kDa while maintaining some control over the polymerization (ĐM < 2).
Herein, we report the optimization of CTA structure for the RAFT/MADIX synthesis of VAc/MDO copolymers. Further study of the polymerization process revealed that loss of control in the polymerization was a result of loss of the xanthate functionality through a Z-group fragmentation mechanism that leads to the formation of carbonodithioate functionality, confirmed by 13C nuclear magnetic resonance (NMR) spectroscopy. Using a p-methoxyphenyl xanthate CTA, greater control over the polymerization was demonstrated with reduced dispersities than have previously been reported and greater chain-end retention. We also report on the first example of homopolymerization of MDO using xanthates. Despite the low conversions, the presence of the CTA also produced poly(MDO) with high end-group retention, as confirmed by 1H NMR and MALDI-ToF MS analysis, and post-polymerization functionalization through aminolysis and Michael addition of propargyl methacrylate to form alkyne functional poly(MDO).
Experimental
Materials and methods
The following chemicals were used as received; alumina, activated basic (Al2O3: Sigma-Aldrich, Brockmann I, standard grade, ∼150 mesh, 58 Å), carbon disulfide (CS2: Fisher Scientific, AR grade), hexylamine (Sigma-Aldrich, 99%), magnesium sulfate (MgSO4: anhydrous, Fisher Scientific, LR grade), methyl 2-bromopropionate (MBP: Sigma-Aldrich, 98%), methyl bromoacetate (MBA: Sigma-Aldrich, 97%), N-methylmaleimide (Sigma-Aldrich, 97%), silica gel (SiO2: Apollo Scientific, 40–63 μm), sodium chloride (NaCl: Fisher Scientific, >99%), sodium hydride (NaH: Sigma-Aldrich, 60 wt% dispersion in mineral oil), sodium hydrogen carbonate (NaHCO3: Fisher Scientific, >99%), triethylamine (Et3N: Fisher Scientific, >99%). The following solvents were used as received; acetone (VWR International, AR grade), chloroform (CHCl3: VWR International, AR grade), d-chloroform (CDCl3: Apollo, >99%), d6-benzene (C6D6, Apollo, >99.5%), dichloromethane (CH2Cl2: VWR International, AR grade), diethyl ether (Fisher Scientific, LR grade), N,N-dimethylformamide (DMF: Sigma-Aldrich, HPLC grade), ethyl acetate (EtOAc: Fisher Scientific, LR grade), 1-hexanol (Acros Organics, 98%), petroleum spirit (BR 40–60 °C, VWR International, AR grade), and 2-propanol (IPA, VWR International, AR grade). Tetrahydrofuran (THF: VWR International, AR grade) was dried using solvent towers. 2,2′-azobis(2-methylpropionitrile) (AIBN: Molekula) and 1,1′-azobis(cyclohexanecarbonitrile) (ABCN: Sigma-Aldrich, 98%) were recrystallized from acetone prior to use. Vinyl acetate (VAc: Sigma-Aldrich, ≥99%) was dried and vacuum distilled over CaH2 to remove the inhibitor and residual water. 2-Methylene-1,3-dioxepane (MDO) was synthesized using the previously described method of Bailey et al.1 then dried and vacuum distilled over CaH2. Both monomers were degassed by freeze–pump–thaw and transferred into a glove-box ready for use. Propargyl methacrylate (Alfa Aesar, 98%) was used as received.O-ethyl-S-ethyl 2-propionylxanthate (CTA 3) was synthesized using the previously described method of Skey et al.43
General considerations
Nuclear Magnetic Resonance spectra were recorded at 400 MHz (1H NMR) and 100 MHz (13C NMR) in CDCl3 on a Bruker DPX-400 spectrometer at 293 K. Chemical shifts are reported as δ in parts per million (ppm) and referenced to the chemical shift of the residual solvent resonances (CDCl31H: δ = 7.26 ppm; 13C: δ = 77.16 ppm). The resonance multiplicities are described as s (singlet), d (doublet), t (triplet), q (quartet) or m (multiplet).Size exclusion chromatography (SEC) analyses were performed on a system composed of a Varian 390-LC-Multi detector using a Varian Polymer Laboratories guard column (PLGel 5 μM, 50 × 7.5 mm), two mixed D Varian Polymer Laboratories columns (PLGel 5μM, 300 × 7.5 mm) and a PLAST RT autosampler. Detection was conducted using a differential refractive index (RI) and an ultraviolet (UV) detector set to 280 nm. The analyses were performed in CHCl3 at 313 K and containing 0.5% w/w triethylamine (Et3N) at a flow rate of 1.0 mL min−1. Polystyrene (PS) (162–2.4 × 105 g mol−1) standards were used to calibrate the system. Molecular weights and dispersities were determined using Cirrus v2.2 SEC software.
IR spectroscopy was carried out using a Perkin Elmer Spectrum 100 FT-IR. 16 scans from 600 to 4000 cm−1 were taken, and the spectra corrected for background absorbance.
Mass spectra were acquired by matrix-assisted laser desorption and ionization time-of-flight mass spectrometry (MALDI-ToF MS) using a Bruker Daltonics Ultraflex Extreme MALDI-ToF mass spectrometer, equipped with a nitrogen laser delivering 3 ns laser pulses at 337 nm. Solutions of DCTB as matrix (30 g L−1), NaTFA (2 g L−1) as cationization agent and polymer (1 g L−1) were prepared in THF. 20 μL aliquots of matrix, polymer and NaTFA solutions were mixed in an Eppendorf tube then applied to the target followed by solvent evaporation to prepare a thin matrix/analyte film. The samples were measured in reflector mode.
Synthetic procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum spirit/EtOAc) afforded the target compound as a pale yellow oil (5.1 g, 48.0%). Rf (9
1 petroleum spirit/EtOAc) afforded the target compound as a pale yellow oil (5.1 g, 48.0%). Rf (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum spirit/EtOAc) 0.38; HRMS m/z theory: 287.0746 (M − Na+); Found: 287.0749; Microanalysis: Calculated for C11H20O3S2: C, 49.97; H, 7.62; Found: C, 50.43; H, 7.66%. 1H NMR (CDCl3): δ 0.90 (t, 3H, 3JH–H = 6.8 Hz, CH3CH2), 1.25–1.48 (m, 6H, CH3(CH2)3CH2), 1.57 (d, 3H, 3JH–H = 7.4 Hz, SCHCH3), 1.78 (m, 2H, 3JH–H = 7.2 Hz, CH2CH2CH2O), 3.75 (s, 3H, (C
1 petroleum spirit/EtOAc) 0.38; HRMS m/z theory: 287.0746 (M − Na+); Found: 287.0749; Microanalysis: Calculated for C11H20O3S2: C, 49.97; H, 7.62; Found: C, 50.43; H, 7.66%. 1H NMR (CDCl3): δ 0.90 (t, 3H, 3JH–H = 6.8 Hz, CH3CH2), 1.25–1.48 (m, 6H, CH3(CH2)3CH2), 1.57 (d, 3H, 3JH–H = 7.4 Hz, SCHCH3), 1.78 (m, 2H, 3JH–H = 7.2 Hz, CH2CH2CH2O), 3.75 (s, 3H, (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)OCH3), 4.41 (q, 1H, 3JH–H = 7.4 Hz, SCHCH3), 4.56 (t, 2H, 3JH–H = 6.7 Hz, CH2CH2CH2O); 13C NMR (CDCl3): δ 212.3, 172.0, 74.7, 52.9, 47.1, 31.5, 28.2, 25.7, 22.64, 17.1, 14.1.
O)OCH3), 4.41 (q, 1H, 3JH–H = 7.4 Hz, SCHCH3), 4.56 (t, 2H, 3JH–H = 6.7 Hz, CH2CH2CH2O); 13C NMR (CDCl3): δ 212.3, 172.0, 74.7, 52.9, 47.1, 31.5, 28.2, 25.7, 22.64, 17.1, 14.1.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)OCH3), 4.04 (s, 2H, SCH2(C
O)OCH3), 4.04 (s, 2H, SCH2(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)O), 6.9–7.1 (Ar, 4H); 13C NMR (CDCl3): δ 213.1, 168.1, 157.9, 148.1, 122.7, 114.5, 55.6, 53.0, 38.7.
O)O), 6.9–7.1 (Ar, 4H); 13C NMR (CDCl3): δ 213.1, 168.1, 157.9, 148.1, 122.7, 114.5, 55.6, 53.0, 38.7.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 petroleum spirit/diethyl ether) afforded the target compound as a light yellow oil (4.6 g, 83.7%). Rf (9
1 petroleum spirit/diethyl ether) afforded the target compound as a light yellow oil (4.6 g, 83.7%). Rf (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Hexane/EtOAc) 0.34; HRMS m/z theory: 245.0277 (M − Na+); Found: 245.0284; Microanalysis: Calculated for C9H16O3S2: C, 43.22; H, 6.35; Found: C, 43.12; H, 6.26%; 1H NMR (CDCl3) δ 1.37 (m, 6H, 3JH–H = 6.0 Hz, (CH3)2CHO), 1.55 (d, 3H, 3JH–H = 7.4 Hz, CH3CHS), 3.73 (s, 3H, CH3OC
1 Hexane/EtOAc) 0.34; HRMS m/z theory: 245.0277 (M − Na+); Found: 245.0284; Microanalysis: Calculated for C9H16O3S2: C, 43.22; H, 6.35; Found: C, 43.12; H, 6.26%; 1H NMR (CDCl3) δ 1.37 (m, 6H, 3JH–H = 6.0 Hz, (CH3)2CHO), 1.55 (d, 3H, 3JH–H = 7.4 Hz, CH3CHS), 3.73 (s, 3H, CH3OC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 4.35 (d, 1H, 3JH–H = 7.4 Hz, CH3CHS), 5.71 (m, 1H, 3JH–H = 6.2 Hz, (CH3)2CHO); 13C NMR (CDCl3): δ 211.2, 172.1, 78.5, 52.8, 46.7, 21.3, 16.9.
O), 4.35 (d, 1H, 3JH–H = 7.4 Hz, CH3CHS), 5.71 (m, 1H, 3JH–H = 6.2 Hz, (CH3)2CHO); 13C NMR (CDCl3): δ 211.2, 172.1, 78.5, 52.8, 46.7, 21.3, 16.9.
General procedure for the synthesis of poly(VAc-co-MDO)
In an inert environment, VAc (1.55 g, 1.8 × 10−2 mol), MDO (0.228 g, 2.0 × 10−3 mol), CTA 2 (55.5 mg, 2.0 × 10−4 mol), ABCN (4.9 mg, 2.0 × 10−5 mol) and C6D6 (15 mol%) were placed into a Young's tapped ampoule and sealed. The solution was subjected to a further 3 freeze–pump–thaw cycles then backfilled with argon. The resulting solution was stirred and heated to 90 °C for 4 h before the polymerization was quenched by plunging the ampoule into an ice bath. An aliquot was taken prior to precipitation for conversion by 1H NMR spectroscopy (CDCl3 was pre-treated by passage through basic Al2O3 to remove any acids present). The polymer was then dissolved in CHCl3 and precipitated several times into hexane until no further monomer residue was observed. The final light yellow solid was dried under vacuum at room temperature for 24 h. Conversion: VAc = 58%; MDO = 51%. SEC (CHCl3 + 0.5% w/w Et3N): Mn = 8.1 kDa, ĐM = 1.38.General procedure for the synthesis of poly(MDO)
In an inert environment, MDO (2.28 g, 2.0 × 10−2 mol), CTA 2 (55.5 mg, 2.0 × 10−4 mol), ABCN (4.9 mg, 2.0 × 10−5 mol) and C6D6 (15 mol%) were placed into a Young's tapped ampoule and sealed. The solution was subjected to a further 3 freeze–pump–thaw cycles then backfilled with argon. The resulting solution was stirred and heated to 90 °C for 24 h before the polymerization was quenched by plunging the ampoule into an ice bath. An aliquot was taken prior to precipitation for conversion by 1H NMR spectroscopy (CDCl3 was pre-treated by passage through basic Al2O3 to remove any acids present). The polymer was then dissolved in CHCl3 and precipitated several times into hexane until no further monomer residue was observed. The final light yellow solid was dried under vacuum at room temperature for 24 h. Conversion: 22%. SEC (CHCl3 + 0.5% w/w Et3N): Mn = 3.9 kDa, ĐM = 1.55.General procedure for in situ aminolysis and Michael addition to form poly(MDO)-S-alkyne
Poly(MDO) derived from CTA 2 (200.0 mg, 7.2 × 10−5 mol, Mn,NMR = 1.7 kDa, ĐM = 1.44), TCEP·HCl (3.0 mg, 1.4 × 10−5 mol), propargyl methacrylate (90.0 mg, 7.3 × 10−4 mol) and DMF (1 mL) were placed into a Schlenk flask and sealed. The solution was then degassed by 3 consecutive freeze–pump–thaw cycles. Hexylamine (47 μL, 3.6 × 10−4 mol) was added under N2 flow and the solution was degassed once more by freeze–pump–thaw, backfilled with N2, and allowed to stir at room temperature for 36 h. The polymer was then precipitated several times from CHCl3 into cold diethyl ether/ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The final pale yellow residue was dried under vacuum at room temperature for 24 h. SEC (CHCl3 + 0.5% w/w Et3N): Mn = 4.0 kDa, ĐM = 1.36.
1). The final pale yellow residue was dried under vacuum at room temperature for 24 h. SEC (CHCl3 + 0.5% w/w Et3N): Mn = 4.0 kDa, ĐM = 1.36.
Results and discussion
We have previously demonstrated the utility of CTA 1 (Fig. 1) for the synthesis of copolymers of VAc and MDO. We noted however that at high incorporations of MDO, the polymers displayed increased dispersity and loss of end-group fidelity at extended times.22 In order to further investigate the mechanism for this loss of end-group and in turn, increase control over the copolymerization and extend the methodology to MDO homopolymerization, we sought to modify the structure of the xanthate chain transfer agent (Fig. 1).We hypothesized that the incorporation of a primary leaving group adjacent to the xanthate, introduced by the ring-opened MDO, forces the xanthate radical intermediate to fragment via the Z-group (Scheme 1), which has previously been observed by Dommanget et al. for xanthate-mediated ethylene polymerizations.45 In an attempt to reduce and even negate this for VAc/MDO copolymerizations, we investigated xanthate design, specifically to target a xanthate in which the Z-group contains a phenyl moiety that would be less likely to stabilize a radical through the proposed Z-group fragmentation mechanism and allow for conventional fragmentation of the dormant polymer chain. To this end, CTA 2, previously reported by Stenzel et al. to polymerize VAc over a range of molecular weights from Mn = 1–50 kDa,44 provided an ideal candidate. CTA 2 was synthesized as reported previously. Optimization of the purification method was required to remove an impurity, identified as the direct addition of MBA onto p-methoxyphenol, running at the same Rf as the product.
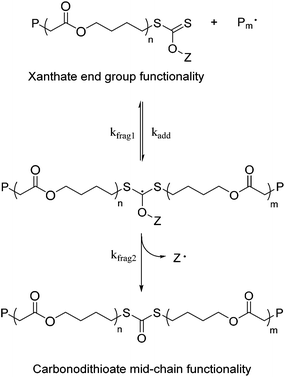 | ||
| Scheme 1 Rearrangement and Z-group fragmentation occurring during the polymerization of MDO in the presence of a xanthate. | ||
VAc/MDO copolymerization
In order to test the efficacy of CTA 2 in the copolymerization of VAc and MDO, we emulated the conditions that we had reported previously.22 Monomer ratios of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 and 30
30 and 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 VAc
70 VAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MDO were polymerized for 24 h at 60 °C in 15 mol% C6D6. Analysis by 1H NMR spectroscopic measurements showed low monomer conversions were achieved, with 70
MDO were polymerized for 24 h at 60 °C in 15 mol% C6D6. Analysis by 1H NMR spectroscopic measurements showed low monomer conversions were achieved, with 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 VAc
30 VAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MDO monomer ratio only reaching 17 and 12% conversion for VAc and MDO respectively, and 30
MDO monomer ratio only reaching 17 and 12% conversion for VAc and MDO respectively, and 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 VAc
70 VAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MDO monomer ratio only reaching monomer conversions of 7% and 4%. We hypothesized this was due to low fragmentation rate of the xanthate from the dormant polymer chain. In an attempt to increase this fragmentation rate and hence monomer conversions, ABCN (Vazo 88) replaced AIBN as the initiating species and the reaction temperature was increased to 90 °C. In this fashion, monomer feed ratios of 90
MDO monomer ratio only reaching monomer conversions of 7% and 4%. We hypothesized this was due to low fragmentation rate of the xanthate from the dormant polymer chain. In an attempt to increase this fragmentation rate and hence monomer conversions, ABCN (Vazo 88) replaced AIBN as the initiating species and the reaction temperature was increased to 90 °C. In this fashion, monomer feed ratios of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 70
10, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, 50
30, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, and 30
50, and 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 for VAc
70 for VAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MDO respectively were polymerized targeting an overall degree of polymerization (DP) of 100 (Table 1, entries 2–5).
MDO respectively were polymerized targeting an overall degree of polymerization (DP) of 100 (Table 1, entries 2–5).
| Time (h) | Monomer feed (VAc/MDO) | Monomer incorp.b (VAc/MDO) | VAc conv. (%) | MDO conv. (%) |
M
obsn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (kDa) c (kDa) |
M
SECn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (kDa) d (kDa) |
M
theorn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e (kDa) e (kDa) |
Đ
M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
|---|---|---|---|---|---|---|---|---|
a Conditions: 15 mol% C6D6, 90 °C, [monomers]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CTA 2] [CTA 2]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [ABCN] = 100 [ABCN] = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1.
b Calculated from 1H NMR spectroscopy.
c Observed molecular weight obtained by 1H NMR spectroscopy end-group analysis, calibrated from aromatic peaks to 4H.
d Observed molecular weight and dispersity obtained by SEC analyses in CHCl3.
e Theoretical molecular weight based on monomer conversion (1H NMR spectroscopy). 0.1.
b Calculated from 1H NMR spectroscopy.
c Observed molecular weight obtained by 1H NMR spectroscopy end-group analysis, calibrated from aromatic peaks to 4H.
d Observed molecular weight and dispersity obtained by SEC analyses in CHCl3.
e Theoretical molecular weight based on monomer conversion (1H NMR spectroscopy).
|
||||||||
| 1.25 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
62 | — | 7.0 | 7.7 | 5.6 | 1.27 |
| 4 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 07 07 |
58 | 51 | 8.2 | 8.1 | 5.4 | 1.38 |
| 15 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
65 | 41 | 8.2 | 7.9 | 5.6 | 1.35 |
| 15 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34 |
55 | 30 | 6.5 | 7.2 | 4.4 | 1.32 |
| 24 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70 |
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54 54 |
55 | 29 | 6.3 | 5.7 | 4.2 | 1.43 |
| 24 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
— | 22 | 4.5 | 3.9 | 2.8 | 1.55 |
As the MDO ratio was increased, longer polymerization times were required to achieve VAc conversions between 55–65%, and MDO conversions were reduced from 51 to 29%. These increased polymerization times are most likely due to the lower fragmentation rate of the xanthate from the dormant polymer chains that have an MDO repeat unit adjacent to the xanthate. These copolymerization kinetic features were similar to a related study conducted by d'Ayala et al. for the copolymerization of VAc with 5,6-benzo-2-methylene-1,3-dioxepane (BMDO, a related CKA monomer).46Mobsn matched well with MSECn but both were consistently higher than Mtheorn, which suggests that some termination occurs during the polymerization. All dispersities (ĐM) for these polymers were within 1.3–1.5, which is typical for xanthate polymerizations as a consequence of their low transfer rates.47 Analysis of molecular weight distributions from SEC (Fig. 2) showed that all copolymers have monomodal distributions, and the overlaid UV traces taken at 280 nm show the presence of the xanthate chain-end throughout the whole distribution. The UV traces also tend to shift towards higher molecular weight with increased MDO content, most likely a result of increasing termination events that result in loss of the xanthate chain-end and lower molecular weight tailing.
As a control, VAc was also homopolymerized (Table 1, entry 1) under the same conditions. The polymerization time was kept to 1.25 h so that the monomer conversion was similar to those in the copolymer syntheses. Analysis of the 1H NMR spectrum and SEC chromatogram showed Mobsn = 7.0 kDa to be in agreement with MSECn = 7.7 kDa, which again was slightly higher than Mtheorn. The monomodal chromatogram displayed a ĐM = 1.27, which is comparable to previous reports of poly(VAc) synthesized this way.44
Analysis of all copolymers by 1H NMR spectroscopy (see ESI†) showed clear evidence of aromatic peaks associated with the Z- group from the xanthate CTA 2 at δ = 6.9–7.0 ppm as well as the methoxy peak at δ = 3.8 ppm from the R-group. A comparison of all 1H NMR spectra (see ESI, Fig. S25†) showed that as the VAc content in the copolymers decreased, the VAc peak intensity at δ = 5.0 ppm also decreased, and the intensity of the peak associated with MDO at δ = 4.2 ppm increased as expected. Also, at higher MDO content, the intensity of VAc-MDO diad at δ = 5.2 ppm increased and the intensity of VAc-VAc diad at δ = 4.9 ppm decreased, which indicates that an increased content of MDO is incorporated into the polymer backbone. However, as the content of MDO increased, there was also evidence of increased side reactions present, with a peak at δ = 6.7 ppm whose intensity was increased in polymers with higher MDO content. We postulate that this resonance occurs as a consequence of the proposed Z-group fragmentation that would result in a p-methoxyphenyl radical that could reinitiate or terminate polymer chains. Additionally, peaks associated with backbiting side reactions at δ = 0.9, 3.15 and 3.65 ppm were observed. There was no evidence of the methine proton of VAc adjacent to the xanthate chain-end, however there was a peak at δ = 3.2 ppm that is consistent with the CH2 resonance from MDO adjacent to a xanthate; the integration of which was approximately 2 for all copolymers. This would suggest that all dormant polymer chains have the xanthate attached to a terminal MDO unit, not to a VAc unit, which would be a result of the slow fragmentation rate from the MDO alkyl chain to form a reactive radical species and is comparable to the observations by Dommanget et al. in the RAFT/MADIX-mediated copolymerization of VA and ethylene.45,48 Further analysis of 13C NMR spectra of the copolymers (see ESI†) also showed evidence of xanthate chain-end retention, in particular peaks associated with the aromatic group (δ = 114, 123, 148 and 158 ppm), the methoxy peak at δ = 56 ppm, as well as the dithiocarbonate peak at δ = 215 ppm. There was also no evidence of an acetal peak at δ = 100 ppm which would arise from the incorporation of ring-retained MDO within the copolymer.
MDO homopolymer synthesis and comparison with other CTAs
As well as conducting VAc/MDO copolymerizations, the homopolymerization of MDO was also trialled using CTA 2, (Table 1, entry 6). Following polymerization under identical conditions as outlined above, SEC and 1H NMR spectroscopic analysis showed Mobsn = 4.5 kDa to be in agreement with MSECn = 3.9 kDa. While this was again slightly higher than Mtheorn, the dispersity remained low, ĐM = 1.55 and the molecular weight distribution was monomodal with a low molar mass tail (Fig. 2). The overlaid RI and UV traces taken at 280 nm showed the presence of the phenyl moiety of the xanthate on the chain-end throughout the whole distribution, albeit shifted to higher molecular weight as a result of the “dead” chains not containing the UV-active xanthate end-group. To further confirm our hypothesis that the suppression of fragmentation of the xanthate radical intermediate via the Z-group can be achieved by incorporation of a phenyl Z-group, three other xanthates were tested in the polymerization of MDO (Fig. 1). CTA 1 was tested as this was the xanthate used in our previous study of VAc/MDO copolymerizations, whereas CTA 3 was used primarily as a control due to its success in mediating the polymerization of LAMs such as vinyl acetate.49,50 Finally, CTA 4 was synthesized incorporating an isopropyl Z-group in an endeavour to increase Z-group fragmentation through the generation of a more stable secondary alkyl radical. Homopolymerizations of MDO mediated with CTAs 1–4 all targeted a final degree of polymerization (DP) of 50 and were reacted for 24 h at 60 °C, with the exception of CTA 2 which was reacted at 90 °C to enhance conversion as no polymerization occurs at 60 °C (Table 2). Monomer conversions were similar for all four xanthates, only varying from 17–21%, and the number-average molecular weights (Mn) determined by SEC varied from 2.8–5.2 kDa. However, poly(MDO) mediated with CTA 4 showed a much broader dispersity of 1.90 compared to that observed for poly(MDO) mediated with CTAs 1 and 3; poly(MDO) mediated with CTA 2 at 90 °C showed the lowest dispersity, ĐM = 1.44. Analysis of the SEC UV chromatograms at 280 nm also showed a very small peak for poly(MDO) mediated with CTA 4, yet 1 and 3 displayed stronger UV responses, which confirms greater retention of the xanthate end-group (Fig. S45†). Again, poly(MDO) mediated with CTA 2 displayed the strongest UV chromatographic peak. These observations were further corroborated by analysis of the 1H NMR spectra for all four polymers (Fig. S30, S35, S40 and S43†) in which poly(MDO) mediated with CTA 2 displayed a very high retention of the xanthate chain-end, whereas poly(MDO) mediated with CTAs 1, 3, and 4 retained a lower amount of Z-group functionality. More in depth analysis of the polymer chain-ends by 13C NMR spectroscopy was able to further probe the retention of xanthate at the chain-end by comparison of the resonance at δ = 214 ppm (attributed to the xanthate carbonyl) with that of a resonance at δ = 189 ppm that is attributed to the formation of a carbonodithioate carbonyl,51,52 consistent with our hypothesis of Z-group fragmentation and electron rearrangement. Most notably, poly(MDO) mediated with CTA 4 displayed a complete loss of the xanthate carbon peak (Fig. S44†) whereas poly(MDO) mediated with CTAs 1, 2 and 3 showed both the xanthate carbonyl peak and carbonodithioate carbonyl peak (Fig. S31, S36 and S41† respectively) with the polymer from CTA 2 resulting in the highest retention of xanthate end-group.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CTA]
[CTA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [AIBN] = 50
[AIBN] = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1)
0.1)
| CTA | MDO conv. (%) |
M
n
obs.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (kDa) b (kDa) |
M
n
SEC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (kDa) c (kDa) |
M
n
theo.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (kDa) d (kDa) |
Đ
M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e e |
UVf |
|---|---|---|---|---|---|---|
| a Polymerization was conducted at 90 °C using ABCN as the initiator. b Observed molecular weight obtained by 1H NMR spectroscopy end-groups analysis. c Observed molecular weight obtained by SEC analyses in CHCl3. d Theoretical molecular weight based on monomer conversion (1H NMR). e Dispersities obtained by SEC analyses in CHCl3. f SEC UV analysis at 280 nm – for SEC chromatograms see Fig. S45. | ||||||
| 1 | 20.0 | 17.7 | 4.2 | 1.4 | 1.55 | Weak |
| 2 | 1.8 | — | — | 0.4 | — | — |
| 2a | 16.7 | 1.7 | 2.8 | 1.1 | 1.44 | Strong |
| 3 | 21.9 | 6.0 | 2.8 | 1.5 | 1.58 | Weak |
| 4 | 21.3 | 9.5 | 5.2 | 1.4 | 1.90 | V. Weak |
Poly(MDO) mediated with CTAs 1 and 2 were also analysed by MALDI-ToF MS in reflector mode to further quantify the level of retention of xanthate on the polymer chain-end. The mass distribution for poly(MDO) mediated with CTA 2 (Fig. S37†) shows a major peak that belongs to sodium-charged poly(MDO) initiated with the R-group and terminated with the Z-group of CTA 2, with each peak separated by the molecular weight of MDO. Also present is a small distribution attributed to ABCN radical initiation and side reactions that occur during the polymerization process (Fig. S38†). In comparison, the mass distribution for poly(MDO) mediated with CTA 1 (Fig. S32†) reveals 3 distinct polymer distributions, with the major distribution corresponding to the carbonodithioate polymer species that arises from Z-group fragmentation of the polymer–polymer radical intermediate during the RAFT process. Also present in this distribution are dormant polymer chains that incorporate the intact CTA (Fig. S33†). These data again corroborate the evidence of p-methoxyphenyl xanthate retention from SEC and 1H NMR spectroscopy.
Post-polymerization functionalization of the terminal xanthate by in situ aminolysis and Michael addition
As final proof of the retention of CTA 2 on the polymer chain-end of poly(MDO)14, an in situ aminolysis and Michael addition experiment was performed using hexylamine and propargyl methacrylate in DMF. This post-polymerization modification was achieved by aminolysis of the xanthate to form the terminal thiol (in the presence of TCEP·HCl to reduce any disulfides that formed) whilst in the presence of propargyl methacrylate that reacts via base-catalyzed Michael addition onto the thiol in situ. SEC analysis shows the peak to be monomodal suggesting no deleterious side reactions occurred during the modification. 1H NMR spectroscopic analysis shows complete removal of the xanthate chain-end with the loss of the aromatic peaks at δ = 7.0 ppm and the methoxy peak at δ = 3.8 ppm (Fig. S47†). This is corroborated by 13C NMR spectroscopy which also shows the loss of the resonance attributed to the thiocarbonyl xanthate at δ = 215 ppm (Fig. S48†). Further evidence of chain-end modification comes from comparison of the MALDI-ToF mass spectra before and after aminolysis/Michael addition (Fig. S37 and S49†). The isotopic mass distribution obtained from reflector mode of the modified poly(MDO) shows the major species present to belong to a sodium-charged poly(MDO) functionalized with propargyl methacrylate added onto the terminal thiol from the xanthate chain-end.Conclusions
In summary, we report the copolymerization of VAc and MDO, as well as the homopolymerization of MDO in the presence of CTA 2. Initial results revealed that this xanthate offers significantly enhanced control over molecular weight and dispersity than other xanthates that have been reported for mediating the rROP of MDO. This xanthate also exhibits high retention onto the polymer chain-end for all copolymers as well as poly(MDO), as demonstrated by MALDI-ToF MS analysis and modification of the chain-end through post-polymerization aminolysis and Michael addition of propargyl methacrylate. MDO homopolymerizations mediated with other known xanthates showed decreased retention of the xanthate chain-end. We were able to confirm that loss of control of the polymerization and loss of end-group fidelity were a result of Z-group fragmentation and rearrangement to form the carbonodithioate functionality.Acknowledgements
The University of Warwick and BP are thanked for co-funding a Ph.D. studentship to G.G.H and the Royal Society and British academy are thanked for the award of a Newton International Fellowship to C.A.B and Industry Fellowship to A.P.D. EPSRC are also acknowledged for funding to support R.K.O. (Career Acceleration Fellowship). NHMRC are thanked for the award of a C.J. Martin Early Career International Fellowship to C.A.B.Notes and references
- W. J. Bailey, Z. Ni and S.-R. Wu, J. Polym. Sci., Part A: Polym. Chem., 1982, 20, 3021–3030 CrossRef CAS PubMed.
- S. Agarwal, Polym. Chem., 2010, 1, 953–964 RSC.
- P. Plikk, T. Tyson, A. Finne-Wistrand and A.-C. Albertsson, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 4587–4601 CrossRef CAS PubMed.
- T. Endo, M. Okawara, W. J. Bailey, K. Azuma, K. Nate and H. Yokono, J. Polym. Sci., Polym. Lett. Ed., 1983, 21, 373–380 CrossRef CAS PubMed.
- W. J. Bailey, Z. Ni and S. R. Wu, Macromolecules, 1982, 15, 711–714 CrossRef CAS.
- W. J. Bailey, S.-R. Wu and Z. Ni, Macromol. Chem. Phys., 1982, 183, 1913–1920 CrossRef CAS PubMed.
- N. Grabe, Y. Zhang and S. Agarwal, Macromol. Chem. Phys., 2011, 212, 1327–1334 CrossRef CAS PubMed.
- Q. Jin, S. Maji and S. Agarwal, Polym. Chem., 2012, 3, 2785–2793 RSC.
- S. Theiler, M. Teske, H. Keul, K. Sternberg and M. Moller, Polym. Chem., 2010, 1, 1215–1225 RSC.
- A. K. Bassi, J. E. Gough, M. Zakikhani and S. Downes, J. Tissue Eng., 2011, 615328 CAS.
- J. Zhou, W. Wang, S. Villarroya, K. J. Thurecht and S. M. Howdle, Chem. Commun., 2008, 5806–5808 RSC.
- M. Labet and W. Thielemans, Chem. Soc. Rev., 2009, 38, 3484–3504 RSC.
- J. N. Hoskins and S. M. Grayson, Polym. Chem., 2011, 2, 289–299 RSC.
- B. Wu and R. W. Lenz, J. Environ. Polym. Degrad., 1998, 6, 23–29 CrossRef CAS.
- W. J. Bailey, T. Endo, B. Gapud, Y. N. Lin, Z. Ni, C. Y. Pan, S. E. Shaffer, S. R. Wu, N. Yamazaki and K. Yonezawa, J. Macromol. Sci., Chem., 1984, A21, 979–995 CrossRef CAS PubMed.
- W. J. Bailey, V. K. Kuruganti and J. S. Angle, ACS Symp. Ser., 1990, 433, 149–160 CrossRef CAS PubMed.
- L. M. Morris, T. P. Davis and R. P. Chaplin, Polymer, 2000, 42, 495–500 CrossRef.
- J. Xu, Z.-L. Liu and R.-X. Zhuo, J. Appl. Polym. Sci., 2007, 103, 1146–1151 CrossRef CAS PubMed.
- V. Delplace, A. Tardy, S. Harrisson, S. Mura, D. Gigmes, Y. Guillaneuf and J. Nicolas, Biomacromolecules, 2013, 14, 3769–3779 CrossRef CAS PubMed.
- V. Delplace, S. Harrisson, A. Tardy, D. Gigmes, Y. Guillaneuf and J. Nicolas, Macromol. Rapid Commun., 2014, 35, 484–491 CrossRef CAS PubMed.
- S. Agarwal, R. Kumar, T. Kissel and R. Reul, Polym. J., 2009, 41, 650–660 CrossRef CAS.
- G. G. Hedir, C. A. Bell, N. S. Ieong, E. Chapman, I. R. Collins, R. K. O'Reilly and A. P. Dove, Macromolecules, 2014, 47, 2847–2852 CrossRef CAS.
- J. Undin, T. Illanes, A. Finne-Wistrand and A.-C. Albertsson, Polym. Chem., 2012, 3, 1260–1266 RSC.
- S. Agarwal, Polym. J., 2007, 39, 163–174 CrossRef CAS.
- G. E. Roberts, M. L. Coote, J. P. A. Heuts, L. M. Morris and T. P. Davis, Macromolecules, 1999, 32, 1332–1340 CrossRef CAS.
- T. Cai, Y. Chen, Y. Wang, H. Wang, X. Liu, Q. Jin, S. Agarwal and J. Ji, Macromol. Chem. Phys., 2014, 215, 1848–1854 CrossRef CAS PubMed.
- S. Louguet, V. Verret, L. Bedouet, E. Servais, F. Pascale, M. Wassef, D. Labarre, A. Laurent and L. Moine, Acta Biomater., 2014, 10, 1194–1205 CrossRef CAS PubMed.
- S. Agarwal and L. Ren, PMSE Prepr., 2009, 100, 89–90 CAS.
- S. Maji, F. Mitschang, L. Chen, Q. Jin, Y. Wang and S. Agarwal, Macromol. Chem. Phys., 2012, 213, 1643–1654 CrossRef CAS PubMed.
- Y. Zhang, A. Aigner and S. Agarwal, Macromol. Biosci., 2013, 13, 1267–1275 CrossRef CAS PubMed.
- A. Galperin, T. J. Long and B. D. Ratner, Biomacromolecules, 2010, 11, 2583–2592 CrossRef CAS PubMed.
- L.-F. Sun, R.-X. Zhuo and Z.-l. Liu, Macromol. Biosci., 2003, 3, 725–728 CrossRef CAS PubMed.
- S. Choi, K. Lee, S. Kwon and H. Kim, J. Supercrit. Fluids, 2006, 37, 287–291 CrossRef CAS PubMed.
- S. Kwon, K. Lee, W. Bae and H. Kim, Polym. J., 2008, 40, 332–338 CrossRef CAS.
- L. F. Sun, R. X. Zhuo and Z. L. Liu, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 2898–2904 CrossRef CAS PubMed.
- A. Tardy, V. Delplace, D. Siri, C. Lefay, S. Harrisson, B. de Fatima Albergaria Pereira, L. Charles, D. Gigmes, J. Nicolas and Y. Guillaneuf, Polym. Chem., 2013, 4, 4776–4787 RSC.
- J. Huang, R. Gil and K. Matyjaszewski, Polymer, 2005, 46, 11698–11706 CrossRef CAS PubMed.
- H. Wickel, S. Agarwal and A. Greiner, Macromolecules, 2003, 36, 2397–2403 CrossRef CAS.
- J.-Y. Yuan, C.-Y. Pan and B. Z. Tang, Macromolecules, 2001, 34, 211–214 CrossRef CAS.
- T. He, Y.-F. Zou and C.-Y. Pan, Polym. J., 2002, 34, 138–143 CrossRef CAS.
- Y. Wei, E. J. Connors, X. Jia and B. Wang, Chem. Mater., 1996, 8, 604–606 CrossRef CAS.
- Y. Wei, E. J. Connors, X. Jia and B. Wang, J. Polym. Sci., Part A: Polym. Chem., 1998, 36, 761–771 CrossRef CAS.
- J. Skey and R. K. O'Reilly, Chem. Commun., 2008, 4183–4185 RSC.
- M. H. Stenzel, L. Cummins, G. E. Roberts, T. P. Davis, P. Vana and C. Barner-Kowollik, Macromol. Chem. Phys., 2003, 204, 1160–1168 CrossRef CAS PubMed.
- C. Dommanget, F. D'Agosto and V. Monteil, Angew. Chem., Int. Ed., 2014, 53, 6683–6686 CrossRef CAS PubMed.
- G. G. d'Ayala, M. Malinconico, P. Laurienzo, A. Tardy, Y. Guillaneuf, M. Lansalot, F. D'Agosto and B. Charleux, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 104–111 CrossRef PubMed.
- D. Taton, M. Destarac and S. Z. Zard, Handbook of RAFT polymerization, John Wiley & Sons, 2008 Search PubMed.
- E. Rizzardo, G. Moad and S. Thang, Handbook of RAFT Polymerization, Wiley-VCH, 2008 Search PubMed.
- S. L. Brown, S. Perrier, C. M. Rayner, A. Cooper, S. Graham and S. Rannard, Chem. Commun., 2007, 2145–2147 RSC.
- C. Barner-Kowollik, T. P. Davis and M. H. Stenzel, Chem. Commun., 2004, 1546–1547 Search PubMed.
- A. R. Katritzky, S. Sobiak and C. M. Marson, Magn. Reson. Chem., 1988, 26, 665–670 CrossRef CAS PubMed.
- C. Copeland and R. V. Stick, Aust. J. Chem., 1984, 37, 1483–1487 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization of all CTAs, homopolymers and copolymers. See DOI: 10.1039/c5py01156f |
| This journal is © The Royal Society of Chemistry 2015 |


