 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effects of UV radiation on aquatic ecosystems and interactions with other environmental factors
Donat-P.
Häder
*a,
Craig E.
Williamson
b,
Sten-Åke
Wängberg
c,
Milla
Rautio
d,
Kevin C.
Rose
e,
Kunshan
Gao
f,
E. Walter
Helbling
g,
Rajeshwar P.
Sinha
h and
Robert
Worrest
i
aEmeritus from Friedrich-Alexander Universität Erlangen-Nürnberg, Dept. Biology, Neue Str. 9, 91096 Möhrendorf, Germany. E-mail: donat@dphaeder.de
bDepartment of Biology, Miami University, Oxford, OH 45056-1400, USA
cDept. Biological and Environmental Science, University of Gothenburg, P.O. Box 461, SE-40530 Göteborg, Sweden
dDépartement des Sciences Fondamentales and Centre for Northern Studies (CEN), Université du Québec à Chicoutimi, Saguenay, Québec, Canada
eDepartment of Zoology, University of Wisconsin, Madison, 250 North Mills Street, Madison, WI 53706, USA
fState Key Laboratory of Marine Environmental Science, Xiamen University (XiangAn Campus, ZhouLongQuan A1-211), XiangAn, Xiamen, Fujian 361102, China
gEstación de Fotobiología Playa Unión, Casilla de Correo 15, (U9103ZAA) Rawson, Chubut, Argentina
hCentre of Advanced Study in Botany, Banaras Hindu University, Varanasi-221005, India
iCIESIN, Columbia University, 190 Turnbull Road, New Hartford, CT 06057-4139, USA
First published on 12th November 2014
Abstract
Interactions between climate change and UV radiation are having strong effects on aquatic ecosystems due to feedback between temperature, UV radiation, and greenhouse gas concentration. Higher air temperatures and incoming solar radiation are increasing the surface water temperatures of lakes and oceans, with many large lakes warming at twice the rate of regional air temperatures. Warmer oceans are changing habitats and the species composition of many marine ecosystems. For some, such as corals, the temperatures may become too high. Temperature differences between surface and deep waters are becoming greater. This increase in thermal stratification makes the surface layers shallower and leads to stronger barriers to upward mixing of nutrients necessary for photosynthesis. This also results in exposure to higher levels of UV radiation of surface-dwelling organisms. In polar and alpine regions decreases in the duration and amount of snow and ice cover on lakes and oceans are also increasing exposure to UV radiation. In contrast, in lakes and coastal oceans the concentration and colour of UV-absorbing dissolved organic matter (DOM) from terrestrial ecosystems is increasing with greater runoff from higher precipitation and more frequent extreme storms. DOM thus creates a refuge from UV radiation that can enable UV-sensitive species to become established. At the same time, decreased UV radiation in such surface waters reduces the capacity of solar UV radiation to inactivate viruses and other pathogens and parasites, and increases the difficulty and price of purifying drinking water for municipal supplies. Solar UV radiation breaks down the DOM, making it more available for microbial processing, resulting in the release of greenhouse gases into the atmosphere. In addition to screening solar irradiance, DOM, when sunlit in surface water, can lead to the formation of reactive oxygen species (ROS). Increases in carbon dioxide are in turn acidifying the oceans and inhibiting the ability of many marine organisms to form UV-absorbing exoskeletons. Many aquatic organisms use adaptive strategies to mitigate the effects of solar UV-B radiation (280–315 nm), including vertical migration, crust formation, synthesis of UV-absorbing substances, and enzymatic and non-enzymatic quenching of ROS. Whether or not genetic adaptation to changes in the abiotic factors plays a role in mitigating stress and damage has not been determined. This assessment addresses how our knowledge of the interactive effects of UV radiation and climate change factors on aquatic ecosystems has advanced in the past four years.
Introduction
Interactions between climate change, ozone, and ultraviolet (UV) radiation are altering exposure to UV radiation in aquatic ecosystems.1,2 Climate change is causing the average global air temperature to rise and precipitation patterns to change, with important consequences for UV exposure in aquatic ecosystems. On a regional scale, changes in climate are highly variable in both space and time, leading to widespread floods in wetter regions, more severe droughts in drier regions, and increases in extreme storm events.3 Climate change is reducing annual snow and ice cover, increasing runoff and concentrations of UV-absorbing dissolved organic matter (DOM) in inland and coastal waters, and increasing the strength of thermal stratification in these systems. Rising atmospheric CO2 concentration induces ocean acidification and alters seawater chemistry and consequently changes UV protection provided by calcified exoskeletons in many aquatic organisms as well as UV exposure levels in aquatic ecosystems.The ecosystem services provided by marine and inland waters include food and drinking water for a growing human population, moderating extreme temperature and weather conditions, and regulating important greenhouse gas concentrations such as that of atmospheric CO2. Freshwater is an indispensible requirement for human existence as well as for all wildlife in terrestrial ecosystems and inland waters. Aquatic ecosystems generate important regional food supplies as well as stimulate regional economies. Fisheries and aquaculture production have increased faster than the world's human population over the last 50 years, constituting an important source of animal protein4 and feeding approximately 1 billion people in Asia alone,5 but these increases may not be sustainable. In the next decade, fish production including that from inland and coastal fish farms is expected to exceed that of other forms of protein.4 Aquatic ecosystems provide other ecosystem services including recreation and tourism, with coral reefs alone estimated to generate US$9.6 billion annually.6 All of these ecosystem services are being influenced by changes in climate and exposure to changing levels of UV radiation.
Here we present an assessment of the advances in our knowledge over the past four years of how interactive effects of climate change and UV radiation are altering aquatic ecosystems, and the critical ecosystem services that they provide.
Consequences of climate change on snow, ice, DOM and exposure to UV radiation
Melting snow and ice: aquatic productivity under high solar radiation
Over the last few decades, rising temperatures have reduced sea and freshwater ice and snow cover with important consequences for underwater exposure to UV radiation. The global ocean temperature has increased by about 1 °C over the last 112 years.7 However, the temperature was almost 2 °C above the average from 1951–1980 in the Arctic8 and the warming of the water along the Antarctic Peninsula has been five times faster than the global average over the past 50 years. One of the reasons for the large temperature increase and drop in ice volume at the poles is an effective feedback mechanism. Ice and snow reflect most solar radiation back into space. In contrast, water and soil absorb most of this radiation, which results in a substantial warming and increased penetration of UV radiation into the ocean water. The higher water temperatures have reduced the Arctic ice cover by 49% during the summer compared to the average during the years between 1979 and 2000.9 The total floating ice volume dropped by about 75% during the same time period. Melting of the Arctic Ocean ice now typically starts in April, 50 days earlier than before the warming,10 and freezing starts in October, about 1 month later than in the past. In recent years Arctic ozone concentrations have decreased, but it is not clear if this trend will continue. It is the first time the O3-depleted area is as large as that in the Antarctic.11 Higher water temperatures resulting in a thinner mixing layer and longer growing season together with increased O3 depletion all have the potential to increase the exposure to UV radiation of aquatic organisms that live in the upper layers of the water column.Of major concern is how climate and UV radiation will alter phytoplankton biomass in the open oceans. Oceanic phytoplankton biomass is important in explaining variations in UV transparency and constitutes a large sink for atmospheric CO2 by taking up a comparable amount of CO2 to all terrestrial ecosystems. Satellite imaging of chlorophyll data shows that phytoplankton concentrations are much higher at polar latitudes than at mid or equatorial regions:12e.g. chlorophyll a concentrations can exceed 20 mg m−3 in the Southern Ocean. Melting ice and snow affect phytoplankton, but local weather and mixing dynamics in the water column contribute differently to the rate and direction of change and are influenced by local weather and mixing dynamics. Judging from 30 years of field studies and satellite chlorophyll fluorescence data, cumulated densities of phytoplankton have decreased by 12% along the West side of the Antarctic Peninsula (Bellingshausen Sea), which has been attributed to increased solar UV-B radiation (280–315 nm) and rapid regional climate change.13 In the north of the Antarctic Peninsula, however, a lower photosynthetic biomass production is attributed to denser cloud cover and the resulting decreased PAR (photosynthetic active radiation, 400–700 nm). In contrast, further south there is less mixing, fewer clouds and consequently lower phytoplankton productivity.13
Increasing cloudiness has been found to limit phytoplankton productivity in the Arctic in open water.14 However, recent research suggests that thinning of the ice is increasing the overall primary productivity and algal biomass in the Arctic. Before recent Arctic warming, the ice cover was about 3 m thick and accumulated over several years, and prevented most light from penetrating into the water below and limited phytoplankton production. Currently the summer ice layer is only about 1 m thick. Pools from meltwater form on the surface, which function as “skylights”. Reduced snowfall further enhances the light availability so that the penetrating solar radiation amounts to about 50% of that incident on the surface.15 This increased light availability fosters a large growth of ice algae and phytoplankton, which is further supported by nutrients upwelling from below. In 2012 a NASA research cruise (ICESCAPE) to the Chukchi Sea off the coast of Alaska reported an unprecedented huge plankton bloom under the Arctic ice extending down to 50 m,16 “as dramatic and unexpected as finding a rainforest in the middle of a desert”.17 This high chlorophyll concentration under the sea ice was not known before and could not be detected by satellite-based remote sensing. Consequently the phytoplankton concentration was among the highest ever recorded extending down to 50 m. Similarly, it was found that these massive blooms occur all over the Arctic Ocean. In the open waters of the Arctic Ocean satellite data have shown a 20% increase in the chlorophyll content between 1998 and 2009.18
Very limited information is available on the effects of changing PAR and exposure to UV radiation on the structure of the food web and total system productivity. In contrast to the discovery of high phytoplankton concentrations under the ice, predictions for the future posit a gradual loss of marine ice algae through loss of sea ice, causing a cascade through the higher trophic levels of the food web. Additionally, meltwater from sea ice and glaciers reduces the salinity, which negatively affects primary producers and the upper levels of the food web.19
Melting sea ice contains about four times more nitrogen than bulk water,20 while increasing PAR results in an increase in the carbon to phosphorous ratio in plankton. Therefore reduced sea ice and increased PAR likely mean that phytoplankton food quality is reduced for herbivorous grazers. Changing ice phenology and light and nutrient availability may also affect species composition. Faster melting of sea ice shifts plankton species toward smaller cell types21 with a better capacity to absorb solar radiation and take up nutrients, which affects the subsequent food web including fish and mammals.
During a 2010 cruise northwest of Svalbard even tropical Radiolaria were found in Arctic waters.22,23 Out of the 145 taxa identified during the cruise, 98 had come from areas much farther south and tropical species were reproducing in their new habitat. Due to the decreased ice cover phytoplankton productivity has extended further north attracting more fish. For example, in the past, capelin – an important prey for Atlantic cod – had a maximal distribution up to 75° N, but capelin were found up to 78° N in 2012 with cod following them.24
Increasing dissolved organic matter and exposure to UV radiation
The increased exposure to PAR and UV radiation caused by the smaller and thinner ice and O3 depletion is partially offset in coastal and inland ecosystems by higher runoff from terrestrial dissolved organic matter (DOM), which decreases water transparency. Increases in global temperature and precipitation (in some areas) are accelerating the release of DOM into lakes, rivers, and coastal oceans.25,26 Strong inshore–offshore gradients in DOM are common to distances of 20 km or more from the shore in large lakes,27 and tens to hundreds of kilometres in the Arctic Ocean (Fig. 1). Remote sensing of this DOM28 provides mechanistic insights into how DOM is changing UV irradiance in coastal and inland waters.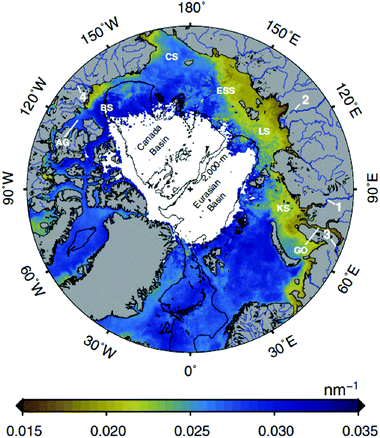 | ||
| Fig. 1 Image of Earth over the North Pole showing the extent of influence of terrestrial DOM inputs from rivers using an optical metric based on UV absorption, called the spectral slope (the slope of the linear relationship between the natural log of the absorption coefficient and wavelength in the 275–295 nm spectral range, nm−1). Although the satellite cannot detect these shorter wavelengths, algorithms have been developed that lead to an accurate relationship between spectral slope in this range and thus DOM source. Browner colours around the periphery of the Arctic Sea show greater inputs of terrestrial DOM, which is particularly pronounced above Eurasia (from ref. 28). | ||
The reasons for the increases in DOM in the Arctic appear to be related primarily to a loss of permafrost. In 2012 a majority of North American and Russian regions reported a 6–10% increase in the depth of soil that thaws annually relative to the average from previous decades.29 The amount of organic carbon stored in permafrost soils is more than twice that in the atmosphere. Thus, when permafrost thaws, large quantities of DOM stored in the soils can be transported to aquatic ecosystems and outgassed as CO2 and/or CH4 after being acted upon by UV radiation and microbes. This DOM is highly photoreactive so exposure to solar UV radiation and visible light accelerate its breakdown and release it to the atmosphere as CO2.30 When permafrost thaws, as much as one third of the organic residues can be converted to CO2 within two weeks.31 The increases in greenhouse gases in turn increase warming and further thawing of permafrost with important consequences for the global radiative balance.
DOM concentrations have more than doubled in many temperate inland waters in recent decades, and climate change has been suggested to play an important role in these increases,32 and is expected to lead to a 65% increase in DOM in boreal waters in the future.25 Increases in precipitation reduce the time that water spends in lakes, which reduces the degradation that occurs when DOM is exposed to UV radiation.33 The frequency of extreme precipitation events such as hurricanes and summer storms has increased in recent years and dramatically increases the export of UV-absorbing terrestrial DOM to aquatic ecosystems. Up to 63% of the annual DOM may come from the top 10% of precipitation events.34 A single hurricane can contribute up to 19% of the annual DOM input to receiving waters35 and increases in DOM associated with a single storm can decrease UV transparency of lake water by a factor of three.36 Lakes can act as buffers of the seasonal variability in DOM input to streams and rivers, thus moderating DOM increases in downstream regions during high flow periods, and increasing DOM concentrations during base flow conditions.37 This buffering of extremes does not seem to influence total annual outflow of DOM from the watershed.38
At the same time DOM inputs contribute to the role of coastal and inland waters in the global carbon cycle. Inland waters are net sources of CO2 to the atmosphere, venting carbon fixed by terrestrial land plants that have subsequently died and decomposed.39 Metabolism and UV-dependent photolysis of terrestrial DOM may have important consequences on greenhouse gas emissions from lakes and for global climate. Inland waters emit large amounts of CH4, a greenhouse gas that is more than 20 times as potent as CO2. The quantity of methane emitted from the world's inland waters is estimated to be equivalent to 25% of the global terrestrial carbon sink40 making inland waters important players in the climate–UV interactions, despite comprising only a fraction (1%) of the total water on Earth.
In addition to the concentration of DOM, the radiation it absorbs influences exposure to UV radiation of inland and coastal waters. Changes in iron (Fe) concentrations, pH, and land-use patterns modify DOM optical characteristics, degradation, and absorption of UV radiation. While the precise reasons for the Fe increase are still not completely understood, there is evidence that waters with high concentrations of soluble Fe are feeding into the surface waters41 and contributing to higher UV absorbance of DOM. Photodegradation of DOM is higher at low pH and high Fe concentrations.42 Peatlands have higher DOM export than do agricultural watersheds and some agricultural landscapes may export more DOM than forested ecosystems.43 The susceptibility of DOM to degradation by UV radiation and visible light (photoreactivity) and microbial decomposition (bioreactivity) varies with the source of the DOM. The DOM from agricultural watersheds is less photoreactive than is DOM from forested lands, but these two types of DOM may be similar in their bioreactivity.44 When exposed to artificial UV lamps in the lab, DOM in water collected from the Chesapeake Bay during base-flow conditions is more photoreactive than that collected during snowmelt in tributaries, but land-use (urban vs. agriculture vs. forested) made little difference.45 These data collectively suggest that land-use patterns may alter not only the amount of DOM in aquatic ecosystems, but also its quality, UV-absorptivity, and subsequent breakdown rates by UV radiation and microbial decomposition to CO2 and CH4.
Apart from reducing the underwater UV radiation and visible light zone, increases in terrestrial DOM alter aquatic food webs via changes in ratios between different basal carbon and nutrient sources. In situ mesocosm studies have demonstrated that increases in nutrient-poor DOM inputs to Arctic lakes will decrease primary productivity and increase heterotrophy (uptake of organic material, in contrast to autotrophy, light driven photosynthesis) within the lake.46 In contrast, addition of DOM with a higher nutrient content to a nutrient-poor alpine lake can stimulate autotrophy more than heterotrophy.47 Increasing DOM also traps heat closer to the surface of aquatic ecosystems, increasing surface temperatures, decreasing the depth of the surface mixed layer and decreasing temperatures in deeper waters. These patterns collectively lead to stronger thermal stratification.48 Because mixing of the water column is reduced, the UV exposure of surface-dwelling organisms may increase or decrease depending on the relative changes in mixing depth versus UV transparency. Further evidence shows that exposure of DOM to solar radiation can lead to the formation of ROS.49,50 Increases in DOM may alter aquatic community structure by altering the temperature of inland and coastal waters, decrease exposure to UV radiation, and ameliorate effects of toxic metals and organic pollutants on fish and other aquatic organisms.51
Collectively these data indicate that climate change-induced inputs of DOM cause severe change in UV transparency and functioning of inland and coastal waters. While DOM provides a refuge from damaging UV radiation for many ecologically and economically important aquatic organisms, it also has the potential to increase the survival of pathogens.52 Higher concentrations of DOM reduce the effectiveness of natural UV radiation on disinfection of drinking water supplies as well as increase its cost and potential for production of carcinogenic disinfection byproducts.53 Understanding the role of interactive effects of DOM, ROS concentrations, UV radiation and climate change in aquatic ecosystems will thus be important for sustaining structure and function of the aquatic ecosystem, for example, through fisheries production and the potential to use the water as a drinking water resource.
Thermal stratification and exposure to UV radiation
Many aquatic organisms, such as zoo- and phytoplankton, are restricted to the upper mixed layer (UML), the lower boundary of which is the thermocline. Temperate latitudes are characterised by seasonal changes in temperature and irradiance, which are reflected in seasonal cycles of abundance and species composition.54,55Tropical waters typically exhibit stable thermal stratification.56 In contrast to polar waters, where the UML can exceed 100 m, in tropical waters it is usually limited to the upper 10–35 m. Across latitudes, nutrient concentrations are higher in deeper layers, but the transport into the mixing zone is limited. As a consequence, these two clearly separated layers shelter distinctly different organisms. At higher latitudes, the input of freshwater from melting ice increases stratification because freshwater is less dense than saltwater.57
Global climate change results in ocean warming, which makes stratification more pronounced and decreases the depth of the UML, causing organisms through all trophic levels to be exposed to increased visible and UV radiation.56,58 In addition, it further limits the transport of nutrients from deeper waters because the lower boundary is more stable.59 Changes in wind speeds are also altering the depth of the UML in many water bodies. Higher temperatures mitigate the inhibitory effects of UV-B radiation by enhancing enzyme-mediated photo-repair as well as photosynthetic carbon fixation and quantum yield.60,61 The molecular mechanism of this enhancement is based on a significantly higher gene expression and activity at 25 °C compared to 20 °C as well as augmented enzyme-driven repair. The mitigating effects of elevated temperatures can reduce the UV stress as has been shown in the South China Sea where the photosynthetic carbon fixation was less inhibited by UV-B radiation in the summer than in the winter.54,62 The respiration index (log of oxygen to carbon dioxide pressure), which may increase with ocean acidification and changes in multiple climate change stressors, could affect photosynthetic production.63 In contrast, higher temperatures can impair the cell cycle resulting in lower growth rates.64
Vertical mixing in the water column largely reduces the UV-induced inhibition of photosynthesis dependent on the mixing frequency and depth, since phytoplankton are constantly moved from the surface to the thermocline and back. Being at the bottom of the UML allows organisms to repair damage that they encounter at the surface, e.g. phytoplankton communities in a coral reef ecosystem where an increased mixing rate and depth results in less UV-B-induced reduction of photosynthetic carbon incorporation.65 UV-A radiation (315–400 nm) can have positive effects on the growth of larger phytoplankton cells under mixing conditions, since this radiation is used by the enzyme photolyase to split UV-B-induced cyclobutane pyrimidine dimers (CPDs). UV-A radiation also contributes to harvesting of photosynthetic energy.62,66
When stratification becomes more pronounced and the mixing layer shallower, hypoxic (low oxygen) areas in inland and coastal waters expand.67 Harmful algal blooms (dinoflagellates and cyanobacteria) can increase in intensity and frequency in both freshwater and marine habitats due to increasing nutrient availability from terrestrial runoff, rising temperatures and increased stratification.68 These organisms are not very sensitive to solar UV-B radiation.
In summary, the increased water temperature due to global climate change reduces the depth of the UML and the organisms dwelling in this layer are exposed to higher UV radiation (Fig. 2). Damage from UV-B radiation encountered at the surface is mitigated by repair processes, which are activated when the organisms are passively transported to the lower boundary of the UML. Higher temperatures favour enzyme-mediated repair of damage by UV radiation.
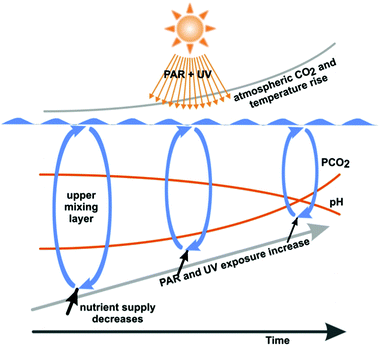 | ||
| Fig. 2 Combined effects of anthropogenic changes in the environmental conditions in marine ecosystems. Increasing atmospheric and water CO2 concentrations reduce the calcifying abilities of many organisms. Increasing water temperatures and incoming solar radiation decrease the depth of the mixing layer (exposing organisms to higher irradiances) and increase the temperature difference between surface and deeper layers. This temperature difference limits the exchange of materials such as nutrients between layers (modified from ref. 56). | ||
Ocean acidification and exposure to UV radiation
The pH of seawater is in the range of 7.5 to 8.4 and is relatively stable due to its buffering capacity. However, increasing atmospheric CO2 concentrations have lowered this value by about 0.1 units, which corresponds to an increase in the H+ concentration by 30%.69 Assuming increasing CO2 emissions (IPCC A1F1 scenario), an atmospheric concentration of 800–1000 ppmv is predicted by 2100, which will correspond to a pH reduction by 0.3–0.4 in the ocean, an increase in H+ ions in surface waters by 100–150%.56 Ocean acidification in conjunction with UV-B radiation affects enzymatic and other biochemical processes in several aquatic organisms, such as phytoplankton, macroalgae and many animals, such as mollusks and corals.70–72 Phytoplankton may serve as a partial remedy to the problem, since they sequester CO2 through photosynthetic carbon fixation.21 While many higher plants benefit from increased atmospheric CO2 concentration, this does not support higher growth rates in phytoplankton.56,73 The red tide microalga, Phaeocystis, showed a much lower growth rate under elevated UV-B which was more pronounced under elevated CO2, indicating that increasing ocean acidification and UV-B act synergistically to reduce photochemical performance.74Zooplankton seem to be little affected by water acidification, although acclimation leads to higher respiration and increased grazing rates.75 Their shells are mainly composed of chitin, which is not affected by acidification. In contrast, those organisms with outer skeletons of calcium carbonate are affected. Increasing acidity affects calcification in phytoplankton, calcified macroalgae76 and animals with exo- or endoskeletons such as corals, depriving these organisms of some defense against solar UV-B radiation.77 UV-B radiation strongly impairs the photosynthetic apparatus in coccolithophorides, while UV-A radiation inhibits calcification.78 The calcified outer scales form a protective exoskeleton.79 Cells grown at high calcium concentrations are more resistant to UV radiation than under limited calcium concentrations.79
In polar regions, dissolution of CO2 from the air into seawater differs from that in low latitude areas. Low sea surface temperature means that more CO2 is dissolved than in low latitude waters. Changes in carbonate chemistry of seawater in the high-latitude oceans are already negatively affecting some species. Consequently, it is projected that within decades, large parts of the polar oceans will become corrosive to the shells of calcareous marine organisms.80 The shells of pteropods, small marine snails (sea butterflies), that are key species in the food web are already dissolving in parts of the Southern Ocean surrounding Antarctica.81 Ocean acidification has effects not only on biological processes but also on the uptake and availability of iron82 and ammonium.55
Degrees of sensitivity of aquatic organisms
Mechanisms of UV radiation damage
In the upper photic zone, aquatic organisms are exposed to solar UV radiation. Although the UV-B irradiance amounts to only a few percent of the total solar radiation, this wavelength band can be hazardous since it affects biomolecules and cellular structures (Fig. 3) and may block enzymatic reactions and interfere with physiological responses such as motility and orientation.69 UV-B radiation can either directly alter biomolecules or induce the formation of reactive oxygen species (ROS) inside the cell, such as singlet oxygen (1O2).83,84 Formation of ROS is augmented by increasing temperatures.85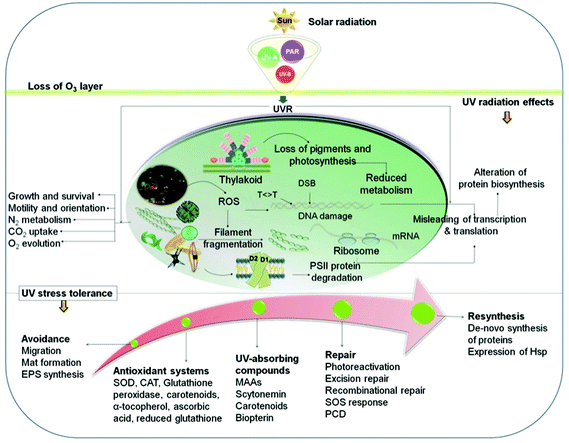 | ||
| Fig. 3 Effects of solar UV radiation on biomolecules, cellular components and physiological responses as well as mitigating strategies and repair mechanisms (for details see text). | ||
Photosynthesis is specifically prone to damage by solar UV-B radiation. In addition to other targets, radiation damages the D1 protein in the electron transport chain of photosystem II (PS II), and is subsequently removed and during repair replaced with a newly synthesized protein.86 Higher water temperatures enhance the repair process, while limited nutrient supply impairs the repair mechanisms.87 An unexpected finding was that UV-B radiation damages phytoplankton more by impairing repair mechanisms than by directly damaging the protein.88 In addition, solar UV-B radiation affects the accessory pigments that funnel solar energy to the reaction centres. The blue pigments, phycobilins, in cyanobacteria and red algae are especially sensitive to damage.89
Another main target of solar UV-B radiation is the DNA in both prokaryotic (bacteria) and eukaryotic (organisms with a cell nucleus) organisms. In addition to single- and double strand breaks and the formation of 6-4 photoproducts (and their Dewar valence isomers), the most frequent lesion induced by UV-B radiation is the induction of CPDs.90 Repair mechanisms for DNA damage include several mechanisms (such as excision repair, mismatch repair and SOS response), but above all the photoactivated CPD photolyase is engaged to break the dimers using the energy of UV-A radiation or blue light photons.90
Mechanisms to avoid or moderate UV-B radiation-induced damage include vertical migration to move out of zones of excessive radiation found in zooplankton and phytoplankton or by mat formation.91 With the exception of very small cells (picoplankton), many aquatic organisms produce UV-absorbing compounds to prevent solar UV-B radiation from damaging the central regions of the cell such as the nucleus. Cyanobacteria synthesize scytonemin to diminish the impact of UV radiation.92 In addition, phytoplankton and macroalgae produce several mycosporine-like amino acids (MAAs).93 UV-B radiation-induced reactive oxygen species are removed by enzymatic reactions and non-enzymatic quenchers including carotenoids.83,94 Animals such as zooplankton are not capable of synthesizing MAAs, but may take up these substances with their food and use them for protection from solar UV radiation.
Several factors make accurate assessment and measurement of the effects of UV radiation on organisms in natural ecosystems very challenging. These factors include the wide variation among organisms in the mechanisms of defense against damage by UV radiation, the need to allow organisms adequate time to adapt to sudden highly elevated UV radiation levels, and assuring accurate measurements of UV radiation. Laboratory experiments with artificial sources of UV radiation often use excessive short wavelength UV-B irradiance levels that are more damaging and have little ecological significance to natural solar radiation due to inappropriate balance in the spectral composition (see Bornman et al.95).
UV radiation exposure levels in aquatic ecosystems exhibit strong gradients over time (daily to annual), depth, and distance from the shore. Different natural populations may vary in their sensitivity to UV radiation over time and may acclimate to the radiation, resulting in some adaptation.
Parasites and pathogens
UV radiation plays an important role in the ecology of many infectious diseases of aquatic organisms, particularly when there is a pronounced difference in the UV radiation tolerance of the host and pathogen or parasite. For example, Metchnikowia is a fungal parasite that is lethal to the important freshwater zooplankton grazer, Daphnia. Relative to its host, the parasite is extremely sensitive to UV radiation and longer wavelength sunlight. Thus in more UV radiation transparent water bodies, outbreaks of this parasite are suppressed and delayed until later in the autumn after incident solar UV radiation has subsided.52 Natural solar radiation is also highly effective at reducing viral infections in some aquatic organisms including viruses of fish and harmful algal bloom (HAB) species. Some experiments that have manipulated natural sunlight reveal a million-fold decrease in the infectious hematopoietic necrosis virus (HNV) in Atlantic salmon in treatments exposing to sunlight versus dark controls for just 3 hours.96 Viruses may be responsible for more than half of the mortality of aquatic cyanobacteria, which has led to interest in using cyanophages to control HABs of toxic cyanobacteria.97 The extreme sensitivity of many viruses to UV radiation damage suggests that knowing more about the changing underwater UV radiation environment may lead to new insights into the potential role of viruses in controlling HAB of cyanobacteria.97Trematodes are some of the most common parasites in intertidal systems. Their larvae are free-living for short periods of time between hosts, which range from snails to other invertebrates and birds. During this short, free-living period in very shallow aquatic environments the larvae may be exposed to high levels of solar UV radiation. Recent UV radiation exposure experiments in the laboratory showed that UV radiation caused DNA damage and oxidative stress in the larvae and showed no evidence of photoprotective MAAs or photoenzymatic repair.98 Similar experiments also demonstrated negative effects of UV-B as well as UV-A radiation exposure on the survival of larvae. In addition, susceptibility to infection of the amphipod secondary host increased when the host was exposed to UV radiation.99 Further experiments on this trematode parasite system revealed significant interaction effects between UV radiation and temperature, with greater UV radiation effects at 20 °C vs. 30 °C.100
Parasites may also play some role in altering the exposure to UV radiation of infected fish hosts. Three-spined stickleback undergo daily vertical migration and are generally deeper in the water column during the day than during the night. However, individuals captured in the surface waters during the day have a higher parasite load than those captured at night, which increases the potential for this parasite to be transmitted to its definitive host – fish-eating birds.101 Increasing evidence is accumulating for the direct and indirect effects of exposure to UV radiation on several marine fish species. Melanosis and melanoma skin cancer rates of up to 15% have been reported in coral trout in the Great Barrier Reef of Australia (Fig. 4).102 The role of UV radiation in the induction of this high prevalence is unknown. In shallow, UV-transparent aquatic ecosystems, such as coral reef flats, photobleaching may lead to destruction of corals and may result in further negative effects when UV radiation and interactions with multiple stressors occurs.103
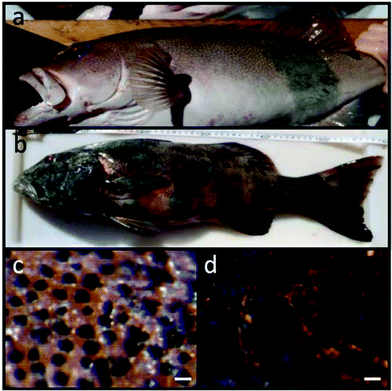 | ||
| Fig. 4 Cancer lesions on coral trout from the Great Barrier Reef observed in up to 15% the natural population and thought to be due to exposure to solar UV radiation. Shown are individuals with only partial (a) and almost full body (b) lesions as well as close-up photographs taken under a microscope of healthy skin (c) and skin lesions (d). Scale bars are 20 μm (from ref. 102). | ||
Bacteria and viruses
Heterotrophic bacteria and viruses are more affected by UV radiation than phytoplankton since they do not synthesize UV-absorbing pigments (but they can repair UV induced damage). Their small dimensions would require extremely large concentrations of these substances to effectively protect them from excessive short-wavelength radiation.104 These organisms respond to UV radiation with adaptive processes such as increased frequency of division, but exposure to UV radiation results in significant changes in species composition due to a varying UV sensitivity of different bacteria taxa.105 Acclimation to long-term UV exposure has also been found in natural sub-Antarctic phytoplankton communities.Inland and coastal waters are rich in bacterioplankton, which feed on the high concentrations of humic substances.106 Bacteria are under pressure from UV-B and UV-A radiation components of sunlight, but recover when the solar radiation decreases during the daily cycle. The highest bacterioplankton activity has been found between 5 and 10 m, below the depths of high solar UV-B radiation exposure. Inhibitory effects of UV radiation on bacterial growth are mitigated by water mixing.107 Different strains have different sensitivity to higher temperatures and UV radiation, leading to selection of more resistant strains and species. Whether or not genetic adaptation may also mitigate the effects of altered stress parameters needs to be examined.108
Phytoplankton
The amount of solar UV and visible radiation determines the species composition of phytoplankton blooms. Large-celled diatoms are less affected by solar UV-B radiation and can utilise high light irradiances.109 In contrast, small-celled phytoplankton such as the ecologically important cyanobacteria and small eukaryotes experience more UV-B-induced damage than larger cells.110In oligotrophic waters (waters with low nutrient contents) solar radiation induces a higher kill rate in marine picoplankton than in larger cyanobacteria and eukaryotic phytoplankton.111 The main driver for mortality is UV-B radiation since filtering out this short wavelength radiation significantly enhances survival. Surface samples are more resistant to solar UV-B radiation than samples collected at depth. Exposing phytoplankton samples experimentally at a fixed depth inhibits photosynthesis more than under natural conditions, where the organisms are moved within the mixing layer.112 The mitigation of the UV-induced inhibition of photosynthetic carbon fixation depends on the mixing rate and depths as shown in tropical coral reef phytoplankton assemblages.65
Phytoplankton of the same taxonomic groups can have significant different sensitivities toward solar UV-B radiation depending on their geographical origin, such as tropical, temperate and Antarctic habitats. In contrast to UV-B radiation, even the highest applied doses of UV-A radiation did not cause growth inhibition. After periods of excessive UV radiation some phytoplankton species, such as the marine diatom Phaeodactylum tricornutum, show a higher growth rate, which partially compensates for prior UV-induced growth reductions. Photosynthesis in phytoplankton is damaged by UV-B radiation mainly at the D1 protein but with higher temperatures increasing the repair rate.87
Primary production in freshwater systems is, in contrast to that in marine systems, often limited by phosphorus. Because of this, the effects of UV radiation on phosphorus metabolism can be important in freshwater aquatic ecosystems. For example, in a high mountain mesocosm experiment, heterotrophic microorganism biomass (bacteria and flagellates) increased when phosphorus addition was reduced by 80% if UV radiation was excluded.113 Sereda et al. investigated how ambient UV radiation affects the phosphorus metabolism of plankton communities from 18 lakes in Ontario and Saskatchewan.114 The turn-over time for phosphorus and the steady state phosphate concentration increased when the organisms were exposed to UV radiation.
The concentration of phytoplankton strongly depends on the pressure by grazers. For example, the seasonal abundance and feeding patterns of copepods in a pelagic food web in the White Sea showed that up to 85% of the daily phytoplankton biomass was consumed by calanoid copepods.115 Exposure to UV radiation affects the quality of phytoplankton in terms of food for zooplanktonic grazers.116 Therefore the level of UV-B radiation is an important modulator of the phytoplankton standing crop.
Mitigation of UV-induced damage by UV-absorbing substances
Phytoplankton use a number of effective repair mechanisms as well as UV-absorbing substances (mostly MAAs) to mitigate UV-B-induced damage of DNA and the photosynthetic apparatus. Samples from phytoplankton blooms under the ozone ‘hole’ counterintuitively show significantly less inhibition of the photosynthetic quantum yield by UV-B radiation than those from outside the ozone-depleted areas, indicating the protective role of their higher MAA concentration.117,118 Another reason for these findings may be that large-size cells occur inside the blooms while outside the blooms smaller cells prevail, which, due to their small size, cannot take advantage of the MAA protection. Ryan et al. experimentally measured the effects of UV-B radiation on sea-ice algae in Antarctica119 and concluded that brine channel communities were better protected from UV-B radiation. They speculate that the high tolerance to UV-B radiation in the brine communities could be due to production of MAAs.Concentrations of UV-absorbing compounds such as MAAs increase with higher exposure to solar radiation in phytoplankton and macroalgae and decrease under experimental conditions without UV.120 In parallel, inhibition by UV radiation of growth and photosynthesis is mitigated with increased content of MAAs. Higher levels of nitrate result in higher contents of MAAs, while phosphate limitation did not affect the MAA content.121 In addition to their UV-absorbing properties, MAAs serve as antioxidants scavenging ROS.122
MAAs are produced in algal cells and protect against UV-B radiation. The production depends on species, degree of impairment, and locality.123 Large differences have been found in MAA production, which correlated with differences in species composition and sensitivity to UV-B radiation. Phytoplankton in inland and coastal waters are generally less tolerant to UV radiation than open ocean assemblages. This is probably because they need less protection and therefore have developed lower MAA concentrations as a consequence of the lower transparency of the water.124 UV-B radiation can impair growth and development, morphology, photosynthesis and nutrient uptake in coastal phytoplankton species.125
In the tropics UV-absorbing MAAs within phytoplankton cells show the same concentration year round, while in temperate waters MAA concentrations are lower in winter than in summer in surface waters <50 m.93 MAA expression is linearly related with the UV irradiance at the surface. In the tropics, phytoplankton is under considerable UV-B radiation stress on sunny days. On cloudy days microplankton (>20 μm) use UV-A radiation as an energy source for photosynthesis, while pico- and nanoplankton are impaired.126 Cloud patterns and density affect the level of UV radiation, but this has rarely been studied in detail. Along a 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km meridional transect (from 52° N to 45° S) the highest MAA concentrations were found in the south (>40° S) and in the north subtropical region.93
000 km meridional transect (from 52° N to 45° S) the highest MAA concentrations were found in the south (>40° S) and in the north subtropical region.93
Interacting stress factors
Satellite monitoring of the oceans shows that phytoplankton concentrations have been declining at about 1% per year over the past 50 years.127 Laufkoetter et al.128 calculated a different number for the decrease (6.5% during the period 1960–2006), simulating phytoplankton net primary production on a global scale with large spatial resolution. However, there are large areas of uncertainty: even though the external factors of temperature, pH, CO2 supply, nutrients, PAR and UV irradiances and mixing depths are known to be primary variables driving photosynthesis and production, their interactions have not been thoroughly investigated.56 This interacting web can only be disentangled by multifactorial analysis and modelling.129,130 In order to reveal the effects in nature with its fast changing temperature, solar radiation and nutrient availability, large scale, long-term studies are needed in the relevant ecosystems.56 This is difficult and time-consuming because of the vast areas to be covered, the diversity of organisms and ecosystems and the low concentrations of cells, especially in open ocean waters. The effects of the many possible feedback mechanisms on marine primary producers are largely unknown. For example, do higher temperatures of the oceans result in denser cloud cover? This could result in lower exposure of the phytoplankton to solar UV radiation and PAR, favouring taxa that are more sensitive to UV radiation.131 Feedback systems have the potential to change the species composition of future assemblages of primary producers with large consequences for inland and marine food webs.Feedback between UV radiation and inland and coastal ecosystem organisms also has the potential to moderate climate. For example, phytoplankton and macroalgae produce dimethylsulfoniopropionate (DMSP),132 which acts as an osmolytic substance or as an antioxidant. DMSP is excreted and broken down to dimethylsulfide (DMS), which enters the atmosphere and down-regulates global warming and reduces the UV-B radiation reaching Earth. In temperate shelf areas, the key phytoplankton species, UV irradiance and nutrient concentrations determine the seasonal cycle of DMS. Ocean acidification reduces DMS accumulation, enhancing global warming, but the role of UV radiation on the production of DMS has not been investigated.133
Increasing environmental pollution, such as crude oil spills, affects algae and bacteria, especially in the Arctic shallow-water marine habitats. Pyrene is a component of crude oil and accumulates in the sediment where it exerts a synergistically negative effect with increased solar UV-B radiation.134 Pyrene is taken up by and concentrated in the cells where it reduces growth rate. In the Greenland Current and Arctic Ocean persistent organic pollutants (POP) accumulate in phytoplankton as documented during the ATOS-ARCTIC cruise on board the R/V Hespérides.135
Exposure to solar UV radiation alters the fatty acid concentration in several phytoplankton groups136 and affects enzyme activity and nitrogen assimilation in both eukaryotic and prokaryotic phytoplankton. However, the sensitivity of phytoplankton species to UV-B radiation is modified by the light history indicating some short-term acclimation to UV-B radiation stress.137 Comparable levels of solar UV-B radiation caused the same degree of growth inhibition in phytoplankton in coastal and offshore surface waters of the South China Sea under clear skies by about 28%. In contrast, inhibition by UV-A radiation was higher in open water samples (13%) than in coastal water samples (4%).66 Due to terrestrial runoff, coastal ecosystems often have higher nutrient concentrations than open ocean systems. In some taxa, such as dinoflagellates, higher nutrient supply (N and P) augments the quantum yield resulting in a different species composition than in open oceanic waters.138 For example, dinoflagellates may outcompete diatoms when rivers deliver larger nutrient loads to coastal waters.139 High nutrient concentrations in coastal waters often induce blooms of toxic phytoplankton (e.g. dinoflagellates), which enter the food chain and can be poisonous to humans. However, solar UV-B radiation does not seem to impair these red tide phytoplankton species.68
The toxicity of many pollutants increases with exposure to UV radiation. Inland and coastal marine environments are under stress from these interactions between UV radiation and pollutants such as polycyclic aromatic hydrocarbons (PAHs), which form from combustion engines, and water-soluble fractions of heavy oil that affect plankton communities. Toxicity of PAHs is enhanced by solar UV radiation as shown with natural phytoplankton communities from the Mediterranean Sea, and Atlantic, Arctic and Southern Oceans.140 Natural phytoplankton communities pre-stressed with UV-B radiation are more susceptible to pollutants, such as atrazine, tributyltin or crude oil, which enter coastal waters from terrestrial drainage or maritime traffic, than those grown under UV radiation-free conditions.
Benthic organisms
Several studies have demonstrated the response of benthic (bottom-dwelling) organisms and communities to solar UV radiation in shallow aquatic habitats. In combined laboratory and field experiments freshwater snails were found to have several mechanisms to avoid damage from UV radiation including behavioural avoidance, photorepair, increased shell thickness, pigmentation and body size.141,142 Juvenile benthic marine polychaetes showed reduced growth and development of tentacles when fed detritus derived from diatoms pre-exposed to artificial UV-B radiation from lamps versus diatoms that were not pre-exposed to UV-B radiation.143 Ostracods, small crustaceans that thrive in shallow benthic habitats, have shells that block 60–80% of UV radiation, as opposed to the exoskeleton of more planktonic Daphnia that block only 35% of UV radiation.144 In a four month-long field experiment on tidal flats where the benthic community was followed when ambient UV-B or UVB + UVA radiation was excluded, the only structural change was a doubling of ostracod biomass under UV radiation compared to PAR only treatments.Seaweeds are an important group of benthic organisms for coastal ecosystems. In addition to being primary producers, seaweeds shape local environments that are important for many animals including fish larvae. Seaweeds are located in fixed positions and need sunlight for photosynthesis and thus cannot escape exposure to high UV radiation. A substantial species-dependent variation in sensitivity to UV radiation correlates with vertical zonation, and smaller and juvenile seaweeds are generally the most sensitive.145 Seaweeds have several life stages of which some are motile (zoospores and gametes) and important for the expansion of the species to new locations. These stages may also be sensitive to DNA damage by solar UV-B radiation as they have only one gene copy.146
The seafloor also harbours benthic communities in the form of invertebrates, bacteria, fungi and microalgae. An experimental study examined the effects of different radiation regimes on the development of these benthic communities in Spitsbergen.147 A total of 17 algal and invertebrate taxa were analysed. No detrimental effects were found from UV radiation (relative to PAR only), although in some species the abundance increased, especially under UV-A radiation. This indicates that at the community level the effects of exposure to UV radiation are dependent on species composition and successional stage. A recent study on the impact of multiple stressors, including UV radiation, temperature and ocean acidification on molluscan development148 showed that the embryos developed significantly better at 26 °C than at 22 °C. Mortality was significantly higher at 22 °C and pH of 7.6. UV radiation had no significant impact on the embryonic development.
Zooplankton
Changes in species composition resulting from climate change149 may favour species that have different UV tolerances. Species that routinely experience high levels of UV radiation are better protected than those that are used to low levels of UV radiation, as was demonstrated by the differences in the UV absorbance of carapaces of Daphnia originating from high UV alpine and low UV boreal lakes.150 In a field survey in Argentina, the relative abundance of more UV-tolerant copepods versus less UV-tolerant Daphnia increased with the distance from the turbid input of a glacier, suggesting UV radiation as a possible regulator of zooplankton community structure, although a role for other factors that changed along the gradient cannot be ruled out.151The response of species to UV radiation varies and is related to the extent of their ability to use various UV avoidance or protection strategies. Evidence confirms that exposure to UV radiation plays an important role in stimulating downward migration of zooplankton during the day in highly transparent waters. These observations have recently been integrated with past studies to develop a more comprehensive theory of daily vertical migration (DVM).152 Some of the strongest evidence for the importance of UV radiation in DVM comes from alpine lakes that lack the visually feeding fish that are often implicated in DVM. For example, in high elevation lakes in Northern Italy153 and Poland,154 crustacean zooplankton migrate to deeper depths during the day in spite of the lack of fish or other visual predators. In the Italian lakes the abundance of crustacean zooplankton in the surface waters of 13 lakes during the day was found to be similar in those lakes with and without fish, suggesting that it is not fish predation that excludes zooplankton from the surface waters of these lakes.155 In temperate lakes of glacial origin in situ experimental manipulation of fish and UV radiation in 15 m deep mesocosms similarly revealed that UV radiation induced stronger downward migration than did the presence of fish.156 In this same study, Daphnia were found to migrate upward in the water column during daylight following a strong storm event that reduced water transparency to UV radiation and visible light. In some marine systems copepods may occur deep enough in the water column that UV radiation plays little or no role in DVM.151 Collectively these observations suggest that changes in water transparency due to climate change are likely to influence the vertical distribution and abundance of zooplankton, a critical link in both freshwater and marine food webs.
The importance of the sublethal effects of UV radiation on both freshwater and marine zooplankton has become increasingly recognized. Sublethal effects of UV radiation on marine copepods include reduced egg quality and survival of larvae.151 In a series of laboratory experiments marine copepods grazed at higher rates on algae that had previously been exposed to elevated levels of UV radiation.157
Further advances have been made in understanding defense mechanisms against UV radiation in freshwater and marine zooplankton, including the trade-offs among multiple defenses and pressures from visual predators and other environmental factors. A recent meta-analysis shows that copepods from freshwater ecosystems have more carotenoids than marine copepods, but that the two groups have similar amounts of MAAs.151 Repair and antioxidant enzymes may similarly provide defense against UV radiation and visual predators simultaneously. Following exposure to artificial UV radiation, freshwater calanoid copepods rapidly activated enzyme systems that reduce peroxidation, cell death, and damage to neurotransmitters.158 Freshwater copepods challenged by simultaneous exposure to fish predation and potentially damaging UV radiation can exhibit trait compensation wherein they increase anti-oxidant enzymes and decrease pigmentation, thus reducing damage by both threats.159 Higher concentrations of carotenoid photoprotective pigments have been observed in copepods in shallow turbid lakes with lower water transparency, apparently due to the lack of aquatic plants and increase in wind-driven turbulence that exposes the copepods to surface UV radiation.160
Fish, amphibians and mammals
Recent evidence indicates that changes in the underwater UV radiation environment may play an important role in regulating invasive fish in cold, clear-water lakes. Studies of two species of invasive warm-water fish (bluegill and largemouth bass) and one native (Lahontan redside) fish in Lake Tahoe, California-Nevada, have demonstrated strong differences in tolerance to UV radiation between native and invasive species. Warm temperatures in shallow near-shore habitats are necessary for the invasive species to breed. High UV transparency in these near-shore habitats prevents the warm-water invasive species from successfully breeding due to the low tolerance to UV radiation of their larvae. Climate change and other disturbances that reduce UV transparency of waters in the warmer shallow shoreline habitat can open an invasion window that permits the invasive warm-water species to become established and reduces native species population sizes through competition and predation.161 The differences in tolerance to UV radiation between invasive and native species can be used in fisheries management to exclude the warm-water invasive species by developing minimum UV attainment thresholds and maintaining high UV transparency of the shoreline breeding habitats.162 Histological studies have demonstrated that species of fish native to highly UV transparent lakes are also more tolerant of simultaneous exposure to solar UV radiation and pollutants such as polycyclic aromatic hydrocarbons (PAHs) than are invasive warm-water species found in characteristically less UV transparent lakes.163 UV radiation and PAH exposure experiments confirmed these results as well as those showing that the native species have more melanin for coping with high UV radiation levels than do the invasive species.164Other recent studies have demonstrated both behavioural avoidance as well as other indirect and direct damaging effects of UV radiation on fish. Experiments that manipulated natural UV radiation with filter foils demonstrated that the survival of freshwater yellow perch larvae is more negatively influenced by UV-A radiation and longer wavelength UV-B and UV-A radiation than by the shorter wavelength UV-B.165 Experimental studies manipulated exposure of two species of salmon fry to solar UV-B radiation in outdoor rearing tanks and tagged smolts using acoustic transmitters to examine their growth rates during rearing as well as subsequent survival rates in the marine environment.166 Exposure to UV-B radiation led to a decrease in early growth of Coho salmon, but had no effects on early survival of either species in the oceans. Exposure of European sea bass larvae to even low levels of artificial UV radiation in the laboratory led to behavioural avoidance, reduced ability to osmoregulate, as well as increased mortality.167 Atlantic cod larvae subjected to prior exposure to artificial UV lamps showed subsequent reductions in the ability to escape suction predators, but not tactile predators;168 feeding rates were also reduced compared to unexposed controls.169 While these studies suggest reductions in feeding will translate to reduced survival under natural conditions,169 this speculation is in contrast to a prior study with largemouth bass larvae under more natural conditions in lakes, where the presence of UV radiation stimulated feeding on zooplankton.170 An analysis of the relationship between brown trout biomass and DOM in 168 lakes in Southern Norway revealed a unimodal relationship with a peak in fish biomass at intermediate DOM levels;171 UV radiation was hypothesized to play a role in decreased biomass at low DOM levels.
In a comprehensive assessment of exposure to UV-B radiation in amphibian breeding habitats, UV-B radiation levels were estimated to be high enough to seriously threaten wood frogs breeding in northern Minnesota vernal pools.172 Reductions in forest canopy cover due to timber harvest as well as changes in water transparency or pool depth related to climate change may further alter exposure to UV radiation in these amphibian breeding habitats.
Marine mammals are being influenced not only by direct damage from exposure to solar UV radiation, but also indirectly through pollution of coastal marine habitats by anthropogenic sunscreen chemicals (sun-tan lotions). They contribute to the bleaching of corals by promoting viral infections and may change the sex of fish.173 Methods for identifying UV-induced skin damage in humans based on real time PCR and mitochondrial DNA (mtDNA) biomarkers have been recently modified and applied to whales.174 Individuals of three different whale species showed significant variations in mtDNA damage in the skin using these techniques. Different species of whales vary in their strategies for coping with exposure to UV radiation. Blue whales, which have relatively pale skin, vary their melanin production seasonally as UV radiation levels vary with their migrations across latitudes; the inverse relationship between melanin and levels of damage from UV radiation suggest that melanin is an effective defense against UV radiation in these cetaceans.175 Sperm whales, which spend more time in the high UV radiation environments at the surface of the ocean all year long, have more melanocytes than blue whales, but similar amounts of melanin.175 The widely used human sunscreen compound, octocrylene, has recently been found in the liver of dolphins off the coast of Brazil.176 Thus it could also have effects on humans.
Gaps in knowledge
While the response of aquatic biomass producers to solar UV-B radiation and global climate change have been characterized to some extent, the effects of interacting stress factors on natural assemblages and ecosystems needs to be further investigated. How the changes in phytoplankton species composition, due to differential sensitivity of individual species caused by UV radiation and interaction with other environmental factors such as temperature, will affect the subsequent food web including fish and mammals also needs to be quantified. There are limited records on the dynamics of overall effects of UV radiation on physical, chemical, and biological attributes of oligotrophic biomass changes.Ocean acidification due to increased atmospheric CO2 concentrations alters the marine chemical environment, which in turn interferes with UV radiation-protecting calcification in many aquatic organisms including phytoplankton, macroalgae and animals such as molluscs and corals. Multifactorial effects including UV-B radiation and ocean acidification on diverse organisms as well as ecosystems should to be studied in order to understand the impacts of future ocean climate changes.
Aquatic organisms employ several lines of defense mechanisms to mitigate the damaging effects of UV radiation. A number of ecologically and economically important organisms need to be screened for the presence of photoprotective compounds and molecular mechanisms of repair.
There is limited knowledge on the cumulative effects of UV radiation–climate change interactions on the nature and type of invasive species and its impact on native populations in aquatic ecosystems.
Acknowledgements
Participation by Prof. Donat-P Häder was supported by the Bundesministerium für Umwelt Naturschutz und Reaktorsicherheit. Participation by Dr Kevin Rose was supported by the Wisconsin Department of Natural Resources (WI DNR) and the North Temperate Lakes Long Term Ecological Research Site (NTL LTER). Participation by Drs Craig Williamson, and Robert Worrest was supported by the U.S. Global Change Research Program. Participation by Prof. Milla Rautio was supported by Canada Research Chair Program.References
- A. F. Bais, R. L. McKenzie, P. J. Aucamp, M. Ilyas, S. Madronich, G. Bernhard and K. Tourpali, Ozone depletion and climate change: Impacts on UV radiation, Photochem. Photobiol. Sci., 2015, 14 10.1039/c4pp90032d , this issue.
- D. J. Erickson III, B. Sulzberger, R. Zepp, A. T. Austin and N. Paul, Effects of stratospheric ozone depletion, solar UV radiation, and climate change on biogeochemical cycling: Interactions and feedbacks, Photochem. Photobiol. Sci., 2015, 14 10.1039/c4pp90036g , this issue.
- IPCC, Summary for Policymakers: Climate change 2013 – The physical science basis, Report No., 2013, pp. 1–38.
- FAO, The state of world fisheries and aquaculture 2012, FAO, Rome, 2012 Search PubMed.
- UNEP, Marine and coastal ecosystems and human well-being: a synthesis report based on the findings of the millennium ecosystem assessment, UNEP Report, Nairobi, 2006 Search PubMed.
- A. V. Parisi and M. G. Kimlin, Personal solar UV exposure measurements employing modified polysulphone with an extended dynamic range, Photochem. Photobiol., 2004, 79, 411–415 CrossRef CAS PubMed.
- M. Fischetti, Deep heat threatens marine life, Sci. Am., 2013, 308, 92 CrossRef PubMed.
- M. New, D. Liverman, H. Schroder and K. Anderson, Four degrees and beyond: the potential for a global temperature increase of four degrees and its implications, Philos. Trans. R. Soc. London, A, 2011, 369, 6–19 CrossRef PubMed.
- J. Zhang, R. Lindsay, A. Schweiger and M. Steele, The impact of an intense summer cyclone on 2012 Arctic sea ice retreat, Geophys. Res. Lett., 2013, 40, 720–726 CrossRef.
- M. Kahru, V. Brotas, B. Manzano-Sarabia and B. G. Mitchell, Are phytoplankton blooms occurring earlier in the Arctic?, Global Change Biol., 2010, 17, 1733–1739 CrossRef.
- G. L. Manney, M. L. Santee, M. Rex, N. J. Livesey, M. C. Pitts, P. Veefkind, E. R. Nash, I. Wohltmann, R. Lehmann, L. Froidevaux, L. R. Poole, M. R. Schoeberl, D. P. Haffner, J. Davies, V. Dorokhov, H. Gernandt, B. Johnson, R. Kivi, E. Kyrö, N. Larsen, P. F. Levelt, A. Makshtas, C. T. McElory, H. Nakajima and M. C. Parrondo, Unprecedented Arctic ozone loss in 2011, Nature, 2011, 478, 469–475 CrossRef CAS PubMed.
- W. Parry, Arctic's Spring Phytoplankton Blooms Arrive Earlier, http://www.livescience.com/13082-arctic-plankton-blooms-ocean-climate-change.html.
- M. Montes-Hugo, S. C. Doney, H. W. Ducklow, W. Fraser, D. Martinson, S. E. Stammerjohn and O. Schofield, Recent changes in phytoplankton communities associated with rapid regional climate change along the Western Antarctic Peninsula, Science, 2009, 323, 1470–1473 CrossRef CAS PubMed.
- S. Bélanger, M. Babin and J.-E. Tremblay, Increasing cloudiness in Arctic damps the increase in phytoplankton primary production due to sea ice receding, Biogeosciences, 2013, 10, 4087–4101 Search PubMed.
- K. E. Frey, D. K. Perovich and B. Light, The spatial distribution of solar radiation under a melting Arctic sea ice cover, Geophys. Res. Lett., 2011, 38, L22501 CrossRef.
- K. R. Arrigo, D. K. Perovich, R. S. Pickart, Z. W. Brown, G. L. van Dijken, K. E. Lowry, M. M. Mills, M. A. Palmer, W. M. Balch, F. Bahr, N. R. Bates, C. Benitez-Nelson, B. Bowler, E. Brownlee, J. K. Ehn, K. E. Frey, R. Garley, S. R. Laney, L. Lubelczyk, J. Mathis, A. Matsuoka, B. G. Mitchell, G. W. K. Moore, E. Ortega-Retuerta, S. Pal, C. M. Polashenski, R. A. Reynolds, B. Schieber, H. M. Sosik, M. Stephens and J. H. Swift, Massive phytoplankton blooms under Arctic sea ice, Science, 2012, 336, 1408 CrossRef CAS PubMed.
- M. J. Viñas, NASA discovers unprecedented blooms of ocean plant life, NASA Sci. News, 2012 Search PubMed.
- K. R. Arrigo and G. L. van Dijken, Secular trends in Arctic Ocean net primary production, J. Geophys. Res.: Oceans, 2011, 116, 160–168 Search PubMed.
- S. Cui, J. He, P. He, F. Zhang, C. Ling and Y. Ma, The adaptation of Arctic phytoplankton to low light and salinity in Kongsfjorden (Spitsbergen), Adv. Polar Sci., 2012, 23, 19–24 CrossRef.
- Y. Zhuang, H. Jin, J. Chen, B. Wang, H. Li, F. Chen, Y. Liu and J. Xu, Nutrient status and phytoplankton-pigments response to ice melting in the Arctic Ocean, Adv. Polar Sci., 2012, 24, 151–158 CAS.
- P. Coupel, H. Y. Jin, M. Joo, R. Horner, H. A. Bouvet, M.-A. Sicre, J.-C. Gascard, J. F. Chen, V. Garçon and D. Ruiz-Pino, Phytoplankton distribution in unusually low sea ice cover over the Pacific Arctic, Biogeosciences, 2012, 9, 4835–4850 CAS.
- http://earth.columbia.edu, http://earth.columbia.edu/articles/view/2993.
- K. R. Bjørklund, S. B. Kruglikova and O. R. Anderson, Modern incursions of tropical Radiolaria into the Arctic Ocean, J. Micropalaeontol., 2012, 31, 139–158 CrossRef.
- P. Renaud, J. Berge, Ø. Varpe, O. Lønne, J. Nahrgang, C. Ottesen and I. Hallanger, Is the poleward expansion by Atlantic cod and haddock threatening native polar cod, Boreogadus saida?, Polar Biol., 2012, 35, 401–412 CrossRef.
- S. Larsen, T. Andersen and D. Hessen, Climate change predicted to cause severe increase of organic carbon in lakes, Global. Change Biol., 2011, 17, 1186–1192 CrossRef.
- H. F. Wilson, J. E. Saiers, P. A. Raymond and W. V. Sobczak, Hydrologic drivers and seasonality of dissolved organic carbon concentration, nitrogen content, bioavailability, and export in a forested New England stream, Ecosystems, 2013, 16, 604–616 CrossRef CAS.
- S. A. Bocaniov, D. R. Barton, S. L. Schiff and R. E. H. Smith, Impact of tributary DOM and nutrient inputs on the nearshore ecology of a large, oligotrophic lake (Georgian Bay, Lake Huron, Canada), Aquat. Sci., 2013, 75, 321–332 CrossRef CAS.
- C. G. Fichot, K. Kaiser, S. B. Hooker, R. M. Amon, M. Babin, S. Bélanger, S. A. Walker and R. Benner, Pan-Arctic distributions of continental runoff in the Arctic Ocean, Sci. Rep., 2013, 3, 1053 Search PubMed.
- V. E. Romanovsky, S. L. Smith, H. H. Christiansen, N. I. Shiklomanov, D. A. Streletskiy, D. S. Drozdov, N. G. Oberman, A. L. Kholodov and S. S. Marchenko, Permafrost, NOAA Report No., 2013, pp. 131–136. http://www.arctic.noaa.gov/reportcard.
- R. M. Cory, B. C. Crump, J. A. Dobkowski and G. W. Kling, Surface exposure to sunlight stimulates CO2 release from permafrost soil carbon in the Arctic, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 3429–3434 CrossRef CAS PubMed.
- J. E. Vonk, P. J. Mann, S. Davydov, A. Davydova, R. G. M. Spencer, J. Schade, W. V. Sobczak, N. Zimov, S. Zimov, E. Bulygina, T. J. Eglinton and R. M. Holmes, High biolability of ancient permafrost carbon upon thaw, Geophys. Res. Lett., 2013, 40, 2689–2693 CrossRef CAS.
- S. Couture, D. Houle and C. Gagnon, Increases of dissolved organic carbon in temperate and boreal lakes in Quebec, Canada, Environ. Sci. Pollut. Res., 2012, 19, 361–371 CrossRef CAS PubMed.
- S. J. Kohler, D. Kothawala, M. N. Futter, O. Liungman and L. Tranvik, In-lake processes offset increased terrestrial inputs of dissolved organic carbon and color to lakes, PLoS One, 2013, 8, e70598 Search PubMed.
- L. B. Knoll, Linking watershed-scale features and processes to carbon, nitrogen, and phosphorus fluxes, PhD thesis, Miami University, Oxford, OH, 2011 Search PubMed.
- G. S. Dhillon and S. Inamdar, Extreme storms and changes in particulate and dissolved organic carbon in runoff: Entering uncharted waters?, Geophys. Res. Lett., 2013, 40 Search PubMed.
- S. Sandro and J. Melack, The effect of an extreme rain event on the biogeochemistry and ecosystem metabolism of an oligotrophic high-elevation lake, Arct. Antarct. Alp. Res., 2012, 44, 222–231 CrossRef.
- K. J. Goodman, M. A. Baker and W. A. Wurtsbaugh, Lakes as buffers of stream dissolved organic matter (DOM) variability: Temporal patterns of DOM characteristics in mountain stream-lake systems, J. Geophys. Res.: Biogeosci., 2011, 116, G00N02 Search PubMed.
- N. R. Lottig, I. Buffam and E. H. Stanley, Comparisons of wetland and drainage lake influences on stream dissolved carbon concentrations and yields in a north temperate lake-rich region, Aquat. Sci., 2013, 75, 619–630 CrossRef CAS.
- L. J. Tranvik, J. Downing, J. B. Cotner, S. Loiselle, R. Striegl, T. J. Ballatore, P. Dillon, K. Finlay, K. Fortino, L. B. Knoll, P. L. Korelainen, T. Kutser, S. Larsen, I. Laurion, D. M. Leech, S. L. McCallister, D. McKnight, J. Melack, E. Overholt, J. A. Porter, Y. Prairie, W. Renwick, F. Roland, B. S. Sherman, D. E. Schindler, S. Sobek, A. Tremblay, M. J. Vanni, A. M. Verschoor, E. v. Wachenfeldt and G. A. Weyhenmeyer, Lakes and reservoirs as regulators of carbon cycling and climate, Limnol. Oceanogr., 2009, 54, 2298–2314 CrossRef CAS.
- D. Bastviken, L. J. Tranvik, J. A. Downing, P. M. Crill and A. A. Enrich-Prast, Freshwater methane emissions offset the continental carbon sink, Science, 2011, 331, 50 CrossRef CAS PubMed.
- E. S. Kritzberg and S. M. Ekström, Increasing iron concentrations in surface waters – a factor behind brownification?, Biogeosciences, 2012, 9, 1465–1478 CrossRef CAS.
- P. Porcal, P. J. Dillon and L. A. Molot, Interaction of extrinsic chemical factors affecting photodegradation of dissolved organic matter in aquatic ecosystems, Photochem. Photobiol. Sci., 2014, 13, 799–812 CAS.
- E. Caverly, J. M. Kaste, G. S. Hancock and R. M. Chambers, Dissolved and particulate organic carbon fluxes from an agricultural watershed during consecutive tropical storms, Geophys. Res. Lett., 2013, 40, 5147–5152 CrossRef CAS.
- Y. H. Lu, J. E. Bauer, E. A. Canuel, Y. Yamashita, R. M. Chambers and R. Jaffe, Photochemical and microbial alteration of dissolved organic matter in temperate headwater streams associated with different land use, J. Geophys. Res.: Biogeosci., 2013, 118, 566–580 CrossRef CAS.
- M. J. Macdonald and E. C. Minor, Photochemical degradation of dissolved organic matter from streams in the western Lake Superior watershed, Aquat. Sci., 2013, 75, 509–522 CrossRef CAS.
- L. Forsstrom, T. Roiha and M. Rautio, Responses of microbial food web to increased allochthonous DOM in an oligotrophic subarctic lake, Aquat. Microb. Ecol., 2013, 68, 171–184 CrossRef.
- C. E. H. Kissman, C. E. Williamson, K. C. Rose and J. E. Saros, Response of phytoplankton in an alpine lake to inputs of dissolved organic matter through nutrient enrichment and trophic forcing., Limnol. Oceanogr., 2013, 58, 867–880 CAS.
- J. S. Read and K. C. Rose, Physical responses of small temperate lakes to variation in dissolved organic carbon concentrations, Limnol. Oceanogr., 2013, 58, 921–931 CAS.
- K. Kadir and K. L. Nelson, Sunlight mediated inactivation mechanisms of Enterococcus faecalis and Escherichia coli in clear water versus waste stabilization pond water, Water Res., 2014, 50, 307–317 CrossRef CAS PubMed.
- A. Silverman, B. M. Peterson, A. B. Boehm, K. McNeill and K. L. Nelson, Sunlight inactivation of human viruses and bacteriophages in coastal waters containing natural photosensitizers, Environ. Sci. Technol., 2013, 47, 1870–1878 CrossRef CAS PubMed.
- A. D. Stasko, J. M. Gunn and T. A. Johnston, Role of ambient light in structuring north-temperate fish communities: potential effects of increasing dissolved organic carbon concentration with a changing climate, Environ. Rev., 2012, 20, 173–190 CrossRef CAS.
- E. P. Overholt, S. H. Hall, C. E. Williamson, C. K. Meikle, M. A. Duffy and C. E. Cáceres, Solar radiation decreases parasitism in Daphnia, Ecol. Lett., 2012, 15, 47–54 CrossRef PubMed.
- S. Haaland, D. Hongve, H. Laudon, G. Riise and R. D. Vogt, Quantifying the drivers of the increasing colored organic matter in boreal surface waters, Environ. Sci. Technol., 2010, 44, 2975–2980 CrossRef CAS PubMed.
- Y. Wu, K. Gao, G. Li and E. W. Helbling, Seasonal impacts of solar UV radiation on photosynthesis of phytoplankton assemblages in the coastal waters of the South China Sea, Photochem. Photobiol., 2010, 86, 586–592 CrossRef CAS PubMed.
- J. M. Beman, C.-E. Chow, A. L. King, Y. Feng, J. A. Fuhrman, A. Andersson, N. R. Bates, B. N. Popp and D. A. Hutchins, Global declines in oceanic nitrification rates as a consequence of ocean acidification, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 208–213 CrossRef CAS PubMed.
- K. Gao, E. W. Helbling, D.-P. Häder and D. A. Hutchins, Responses of marine primary producers to interactions between ocean acidification, solar radiation, and warming, Mar. Ecol.: Prog. Ser., 2012, 470, 167–189 CrossRef CAS.
- M. Steinacher, F. Joos, T. L. Froelicher, L. Bopp, P. Cadule, V. Cocco, S. C. Doney, M. Gehlen, K. Lindsay, J. K. Moore, B. Schneider and J. Segschneider, Projected 21st century decrease in marine productivity: a multi-model analysis, Biogeosciences, 2010, 7, 979–1005 CrossRef CAS.
- J. Turner, N. E. Barrand, T. J. Bracegirdle, P. Convey, D. A. Hodgson, M. Jarvis, A. Jenkins, G. Marshall, M. P. Meredith, H. Roscoe, J. Shanklin, J. French, H. Goosse, M. Guglielmin, J. Gutt, S. Jacobs, M. C. Kennicutt II, V. Masson-Delmotte, P. Mayewski!, F. Navarro, S. Robinson, T. Scambos, M. Sparrow, C. Summerhayes, K. Speer and A. Klepikov, Antarctic climate change and the environment: an update, Cambridge University Press, Report No., Cambridge, 2013, pp. 1–23.
- G. T. Taylor, F. E. Muller-Karger, R. C. Thunell, M. I. Scranton, Y. Astor, R. Varela, L. T. Ghinaglia, L. Lorenzoni, K. A. Fanning and S. Hameed, Ecosystem responses in the southern Caribbean Sea to global climate change, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 19315–19320 CrossRef CAS PubMed.
- E. W. Helbling, A. G. Buma, P. Boelen, H. J. Van der Strate, M. V. Fiorda Giordanino and V. E. Villafañe, Increase in Rubisco activity and gene expression due to elevated temperature partially counteracts ultraviolet radiation-induced photoinhibition in the marine diatom Thalassiosira weissflogii, Limnol. Oceanogr., 2011, 56, 1330–1342 CrossRef CAS.
- S. R. Halac, V. E. Villafañe and E. W. Helbling, Temperature benefits the photosynthetic performance of the diatoms Chaetoceros gracilis and Thalassiosira weissflogii when exposed to UVR, J. Photochem. Photobiol., B, 2010, 101, 196–205 CrossRef CAS PubMed.
- G. Li and K. Gao, Variation in UV irradiance related to stratospheric ozone levels affects photosynthetic carbon fixation of winter phytoplankton assemblages from surface coastal water of the South China Sea, Mar. Biol. Res., 2012, 8, 670–676 CrossRef.
- K. Gao and D. Campbell, Photophysiological responses of marine diatoms to elevated CO2 and decreased pH: a review, Funct. Plant Biol., 2014, 41, 449–459 CrossRef CAS.
- M. Thyssen, G. Ferreyra, S. Moreau, I. Schloss, M. Denis and S. Demers, The combined effect of ultraviolet B radiation and temperature increase on phytoplankton dynamics and cell cycle using pulse shape recording flow cytometry, J. Exp. Mar. Biol. Ecol., 2011, 406, 95–107 CrossRef CAS.
- G. Li, Z. W. Che and K. Gao, Photosynthetic carbon fixation by tropical coral reef phytoplankton assemblages: a UVR perspective, Algae, 2013, 28, 281–288 CrossRef CAS.
- G. Li, K. Gao and G. Gao, Differential impacts of solar UV radiation on photosynthetic carbon fixation from the coastal to offshore surface waters in the South China Sea, Photochem. Photobiol., 2011, 87, 329–334 CrossRef CAS PubMed.
- R. F. Keeling, A. Körtzinger and N. Gruber, Ocean deoxygenation in a warming world, Ann. Rev. Mar. Sci., 2010, 2, 199–229 CrossRef PubMed.
- G. M. Hallegraeff, Ocean climate change, phytoplankton community responses, and harmful algal blooms: a formidable predictive challenge, J. Phycol., 2010, 46, 220–235 CrossRef CAS.
- Richa, R. P. Sinha and D.-P. Häder, Phytoplankton productivity in a changing global climate, in Phytoplankton: Biology, Classification and Environmental Impacts, ed. M. T. Sebastiá, Nova Science Publishers, New York, 2014, pp. 1–35 Search PubMed.
- O. Hoegh-Guldberg, Dangerous shifts in ocean ecosystem function?, ISME J., 2010, 4, 1090–1092 CrossRef PubMed.
- R. Terrado, K. Scarcella, M. Thaler, W. F. Vincent and C. Lovejoy, Small phytoplankton in Arctic seas: vulnerability to climate change, Biodiversity Conserv., 2013, 14, 2–18 CrossRef.
- L. Beaufort, I. Probert, T. De Garidel-Thoron, E. M. Bendif, D. Ruiz-Pino, N. Metzl, C. Goyet, N. Buchet, P. Coupel, M. Grelaud, B. Rost, R. E. M. Rickaby and C. de Vargas, Sensitivity of coccolithophores to carbonate chemistry and ocean acidification, Nature, 2011, 476, 80–83 CrossRef CAS PubMed.
- K. Gao, J. Xu, G. Gao, Y. Li, D. A. Hutchins, B. Huang, Y. Zheng, P. Jin, X. Cai, D.-P. Häder, W. Li, K. Xu, N. Liu and U. Riebesell, Rising carbon dioxide and increasing light exposure act synergistically to reduce marine primary productivity, Nat. Clim. Change, 2012, 2, 519–523 CAS.
- S. Chen and K. Gao, Solar ultraviolet radiation and CO2-induced ocean acidification interacts to influence the photosynthetic performance of the red tide alga Phaeocystis globosa (Prymnesiophyceae), Hydrobiologia, 2011, 675, 105–117 CrossRef CAS.
- W. Li and K. S. Gao, A marine secondary producer respires and feeds more in a high CO2 ocean, Mar. Pollut. Bull., 2012, 64, 699–703 CrossRef CAS PubMed.
- K. Gao and Y. Zheng, Combined effects of ocean acidification and solar UV radiation on photosynthesis, growth, pigmentation and calcification of the coralline alga Corallina sessilis (Rhodophyta), Global. Change Biol., 2010, 16, 2388–2398 CrossRef.
- K. R. N. Anthony, J. A. Maynard, G. Diaz-Pulido, P. J. Mumby, P. A. Marshall, L. Cao and O. Hoegh-Guldenberg, Ocean acidification and warming will lower coral reef resilience, Global. Change Biol., 2011, 17, 1798–1808 CrossRef.
- W. Guan and K. Gao, Impacts of UV radiation on photosynthesis and growth of the coccolithophore Emiliania huxleyi (Haptophyceae), Environ. Exp. Bot., 2010, 67, 502–508 CrossRef CAS.
- K. Xu, K. Gao, V. Villafañe and E. Helbling, Photosynthetic responses of Emiliania huxleyi to UV radiation and elevated temperature: roles of calcified coccoliths, Biogeosciences, 2011, 8, 1441–1452 CrossRef CAS.
- W. Broadgate, U. Riebesell, C. Armstrong, P. Brewer, K. Denman, R. Feely, K. Gao, J.-P. Gattuso, K. Isensee and J. Kleypas, Ocean acidification summary for policymakers–Third symposium on the ocean in a high-CO2 world, 2013 Search PubMed.
- N. Bednaršek, G. A. Tarling, D. C. E. Bakker, Z. S. Fielding, E. M. Jones, H. J. Venables, P. Ward, A. Kuzirian, B. Lézé, R. A. Feely and E. J. Murphy, Extensive dissolution of live pteropods in the Southern Ocean, Nat. Geosci., 2012, 5, 881–885 CrossRef.
- D. Shi, Y. Xu, B. M. Hopkinson and F. M. M. Morel, Effect of ocean acidification on iron availability to marine phytoplankton, Science, 2010, 327, 676–679 CrossRef CAS PubMed.
- Y. Zhang, H.-B. Jiang and B.-S. Qiu, Effects of UVB radiation on competition between the bloom-forming cyanobacterium Microcystis aeruginosa and the chlorophyceae Chlamydomonas microsphaera, J. Phycol., 2013, 49, 318–328 CrossRef CAS.
- S. P. Singh, R. P. Rastogi, R. P. Sinha and D.-P. Häder, Temporal dynamics of ROS biogenesis under simulated solar radiation in the cyanobacterium Anabaena variabilis PCC 7937, Protoplasma, 2014, 251, 1223–1230 CrossRef CAS PubMed.
- R. Muller, C. Desel, F. S. Steinhoff, C. Wiencke and K. Bischof, UV-radiation and elevated temperatures induce formation of reactive oxygen species in gametophytes of cold-temperate/Arctic kelps (Laminariales, Phaeophyceae), Phycol. Res., 2012, 60, 27–36 CrossRef.
- D.-P. Häder, E. W. Helbling, C. E. Williamson and R. C. Worrest, Effects of UV radiation on aquatic ecosystems and interactions with climate change, Photochem. Photobiol. Sci., 2011, 10, 242–260 Search PubMed.
- Y. Li, K. Gao, V. Villafañe and E. Helbling, Ocean acidification mediates photosynthetic response to UV radiation and temperature increase in the diatom Phaeodactylum tricornutum, Biogeosci. Discuss., 2012, 9, 7197–7226 CrossRef.
- D. Wu, Q. Hu, Z. Yan, W. Chen, C. Yan, X. Huang, J. Zhang, P. Yang, H. Deng and J. Wang, Structural basis of ultraviolet-B perception by UVR8, Nature, 2012, 484, 214–219 CrossRef PubMed.
- S. P. Singh, D.-P. Häder and R. P. Sinha, Cyanobacteria and ultraviolet radiation (UVR) stress: mitigation strategies, Ageing Res. Rev., 2010, 9, 79–90 CrossRef CAS PubMed.
- Richa, R. P. Sinha and D.-P. Häder, Physiological aspects of UV-excitation of DNA, in Photoinduced Phenomena in Nucleic Acids, ed. B. Barbatti, A. C. Borin and A. C. Ullrich, Springer, Berlin, Heidelberg, 2014, pp. 1–46 Search PubMed.
- B. B. Barnes, C. Hu, J. P. Cannizzaro, S. E. Craig, P. Hallock, D. L. Jones, J. C. Lehrter, N. Melo, B. A. Schaeffer and R. Zepp, Estimation of diffuse attenuation of ultraviolet light in optically shallow Florida Keys waters from MODIS measurements, Rem. Sens. Environ., 2014, 140, 519–532 CrossRef.
- R. P. Rastogi, R. P. Sinha and A. Incharoensakdi, Partial characterization, UV-induction and photoprotective function of sunscreen pigment, scytonemin from Rivularia sp. HKAR-4, Chemosphere, 2013, 93, 1874–1878 CrossRef CAS PubMed.
- C. A. Llewellyn, D. A. White, V. Martinez-Vincente, G. Tarran and T. J. Smyth, Distribution of mycosporine-like amino acids along a surface water meridional transect of the Atlantic, Micro. Ecol., 2012, 64, 320–333 CrossRef CAS PubMed.
- G. Singh, P. K. Babele, R. P. Sinha, M. B. Tyagi and A. Kumar, Enymatic and non-enzymatic defense mechanisms against ultraviolet-B radiation in two Anabaena species, Process Biochem., 2013, 48, 796–802 CrossRef CAS.
- J. F. Bornman, P. W. Barnes, S. A. Robinson, C. L. Ballaré, S. D. Flinte and M. M. Caldwell, Solar ultraviolet radiation and ozone depletion-driven climate change: effects on terrestrial ecosystems, Photochem. Photobiol. Sci., 2015, 14 10.1039/c4pp90034k , this issue.
- K. A. Garver, A. A. M. Mahony, D. Stucchi, J. Richard, C. Van Woensel and M. Foreman, Estimation of parameters influencing waterborne transmission of infectious Hematopoietic Necrosis Virus (IHNV) in Atlantic Salmon (Salmo salar), PLoS One, 2013, 8, e82296 Search PubMed.
- S. A. A. Jassim and R. G. Limoges, Impact of external forces on cyanophage-host interactions in aquatic ecosystems, World J. Micrbiol. Biotechnol, 2013, 29, 1751–1762 CrossRef PubMed.
- A. Studer, V. M. Cubillos, M. D. Lamare, R. Poulin and D. J. Burritt, Effects of ultraviolet radiation on an intertidal trematode parasite: An assessment of damage and protection, Int. J. Parasitol., 2012, 42, 453–461 CrossRef CAS PubMed.
- A. Studer, M. D. Lamare and R. Poulin, Effects of ultraviolet radiation on the transmission process of an intertidal trematode parasite, Parasitology, 2012, 139, 537–546 CrossRef CAS PubMed.
- A. Studer and R. Poulin, Cercarial survival in an intertidal trematode: a multifactorial experiment with temperature, salinity and ultraviolet radiation, Parasitol. Res., 2013, 112, 243–249 CrossRef CAS PubMed.
- T. N. Quinn, N. W. Kendall, H. B. Rich Jr. and B. E. Chasco, Diel vertical movements, and effects of infection by the cestode Schistocephalus solidus on daytime proximity of three-spined sticklebacks Gasterosteus aculeatus to the surface of a large Alaskan lake, Oecologia, 2012, 168, 43–51 CrossRef CAS PubMed.
- M. Sweet, N. Kirkham, M. Bendall, L. Currey, J. Bythell and M. Heupel, Evidence of melanoma in wild marine fish populations, PLoS One, 2012, 7, e41989 CAS.
- A. R. Harborne, The ecology, behaviour and physiology of fishes on coral reef flats, and the potential impacts of climate change, J. Fish Biol., 2013, 83, 417–447 CrossRef CAS PubMed.
- X. Yuan, K. Yin, P. J. Harrison and J. Zhang, Phytoplankton are more tolerant to UV than bacteria and viruses in the northern South China Sea, Aquat. Microbiol. Ecol., 2011, 65, 117–128 CrossRef.
- J. M. Manrique, A. Y. Calvo, S. R. Halac, V. E. Villafañe, L. R. Jones and E. W. Helbling, Effects of UV radiation on the taxonomic composition of natural bacterioplankton communities from Bahía Engaño (Patagonia, Argentina), J. Photochem. Photobiol., B, 2012, 117, 171–178 CrossRef CAS PubMed.
- C. Romera-Castillo, H. Sarmento, X. A. Álvarez-Salgado, J. M. Gasol and C. Marrasé, Net production and consumption of fluorescent colored dissolved organic matter by natural bacterial assemblages growing on marine phytoplankton exudates, Appl. Environ. Microbiol., 2011, 77, 7490–7498 CrossRef CAS PubMed.
- R. Bertoni, W. H. Jeffrey, M. Pujo-Pay, L. Oriol, P. Conan and F. Joux, Influence of water mixing on the inhibitory effect of UV radiation on primary and bacterial production in Mediterranean coastal water, Aquat. Sci., 2011, 73, 377–387 CrossRef.
- R. P. Goldman and M. Travisano, Experimental evolution of ultraviolet radiation resistance in Escherichia coli, Evolution, 2011, 65, 3486–3498 CrossRef PubMed.
- T. Key, A. McCarthy, D. A. Campbell, C. Six, S. Roy and Z. V. Finkel, Cell size trade-offs govern light exploitation strategies in marine phytoplankton, Environ. Microbiol., 2010, 12, 95–104 CrossRef CAS PubMed.
- M. Llabrés, S. Agustí, P. Alonso-Laita and G. Herndl, Synechococcus and Prochlorococcus cell death induced by UV radiation and the penetration of lethal UVR in the Mediterranean Sea, Mar. Ecol.: Prog. Ser., 2010, 399, 27–37 CrossRef.
- M. Llabrés, S. Agustí, M. Fernández, A. Canepa, F. Maurin, F. Vidal and C. M. Duarte, Impact of elevated UVB radiation on marine biota: a meta-analysis, Global Ecol. Biogeogr., 2013, 22, 131–144 CrossRef.
- P. Jin, K. Gao, V. Villafañe, D. Campbell and W. Helbling, Ocean acidification alters the photosynthetic responses of a coccolithophorid to fluctuating ultraviolet and visible radiation, Plant Physiol., 2013, 162, 2084–2094 CrossRef CAS PubMed.
- M. Medina-Sanchez, J. A. Delgado-Molina, J. Bratbak, G. J. Bullejos, F. Villar-Argaiz and M. Carrillo, Maximum in the middle: Nonlinear response of microbial plankton to ultraviolet radiation and phosphorus, PLoS One, 2013, 8 Search PubMed.
- J. M. Sereda, D. M. Vandergucht and J. J. Hudson, In situ UVA exposure modulates change in the uptake of radiophosphate in size-fractionated plankton assemblages following UVR exposure, Micro. Ecol., 2012, 63, 751–760 CrossRef CAS PubMed.
- D. M. Martynova, N. A. Kazus, U. V. Bathmann, M. Graeve and A. A. Sukhotin, Seasonal abundance and feeding patterns of copepods Temora longicornis, Centropages hamatus and Acartia spp. in the White Sea (66°N), Polar Biol., 2011, 34, 1175–1195 CrossRef.
- S. Nahon, F. Charles, F. Lantoine, G. Vétion, K. Escoubeyrou, M. Desmalades and A. M. Pruski, Ultraviolet radiation negatively affects growth and food quality of the pelagic diatom Skeletonema costatum, J. Exp. Mar. Biol. Ecol., 2010, 383, 164–170 CrossRef.
- A. U. Bracher and C. Wiencke, Simulation of the effects of naturally enhanced UV radiation on photosynthesis of Antarctic phytoplankton, Mar. Ecol.: Prog. Ser., 2000, 196, 127–141 CrossRef CAS.
- K. Oubelkheir, L. A. Clementson, G. F. Moore and G. H. Tilstone, Production of mycosporine-like amino acids by phytoplankton under ultraviolet radiation exposure in the Sub-Antarctic Zone south of Tasmania, Mar. Ecol.: Prog. Ser., 2013, 494, 41–63 CrossRef CAS.
- K. G. Ryan, A. McMinn, E. N. Hegseth and S. K. Davy, The effects of ultraviolet radiation on Antarctic sea-ice algae, J. Phycol., 2012, 48, 74–84 CrossRef CAS.
- S. P. Singh, S.-Y. Ha, R. P. Sinha and D.-P. Häder, Photoheterotrophic growth unprecedentedly increases the biosynthesis of mycosporine-like amino acid shinorine in the cyanobacterium Anabaena sp., isolated from hot springs of Rajgir (India), Acta Physiol. Plant., 2014, 36, 389–397 CrossRef CAS.
- Z. Xu and K. Gao, Impacts of UV radiation on growth and photosynthetic carbon acquisition in Gracilaria lemaneiformis (Rhodophyta) under phosphorus-limited and replete conditions, Funct. Plant Biol., 2009, 36, 1057–1064 CrossRef CAS.
- C. A. Llewellyn and R. L. Airs, Distribution and abundance of MAAs in 33 species of microalgae across 13 classes, Mar. Drugs, 2010, 8, 1273–1291 CrossRef CAS PubMed.
- S. Y. Ha, Y. M. Kim, M. O. Park, S. H. Kang, H. C. Kim and K. H. Shin, Production of mycosporine-like amino acids of in situ phytoplankton community in Kongsfjorden, Svalbard, Arctic, J. Photochem. Photobiol., B, 2012, 114, 1–14 CrossRef CAS PubMed.
- L. M. Ayoub, P. Hallock, P. G. Coble and S. S. Bell, MAA-like absorbing substances in Florida Keys phytoplankton vary with distance from shore and CDOM: Implications for coral reefs, J. Exp. Mar. Biol. Ecol., 2012, 420, 91–98 CrossRef.
- M. Fiorda Giordanino, S. M. Strauch, V. E. Villafañe and E. W. Helbling, Influence of temperature and UVR on photosynthesis and morphology of four species of cyanobacteria, J. Photochem. Photobiol., B, 2011, 103, 68–77 CrossRef PubMed.
- G. Li and K. Gao, Cell size-dependent effects of solar UV radiation on primary production in coastal waters of the South China Sea, Estuaries Coasts, 2013, 36, 728–736 CrossRef CAS.
- D. G. Boyce, M. R. Lewis and B. Worm, Global phytoplankton decline over the past century, Nature, 2010, 466, 591–596 CrossRef CAS PubMed.
- C. Laufkoetter, M. Vogt and N. Gruber, Trends in marine plankton composition and export production in a CCSM-BEC hindcast (1960–2006), in EGU General Assembly Conference Abstracts, 2013, vol. 15, p. 11917 Search PubMed.
- P. W. Boyd, Beyond ocean acidification, Nat. Geosci., 2011, 4, 273–274 CrossRef CAS.
- D.-P. Häder, Does enhanced solar UV-B radiation affect marine primary producers in their natural habitats?, Photochem. Photobiol., 2011, 87, 263–266 CrossRef PubMed.
- I. E. Huertas, M. Rouco, V. López-Rodas and E. Costas, Warming will affect phytoplankton differently: evidence through a mechanistic approach, Proc. R. Soc. London, B, 2011, 278, 3534–3543 CrossRef PubMed.
- C. Ruiz-González, M. Galí, E. Sintes, G. J. Herndl, J. M. Gasol and R. Simó, Sunlight effects on the osmotrophic uptake of DMSP-sulfur and leucine by Polar phytoplankton, PLoS One, 2012, 7, e45545 Search PubMed.
- K. D. Six, S. Kloster, T. Ilyina, S. D. Archer, K. Zhang and E. Maier-Reimer, Global warming amplified by reduced sulphur fluxes as a result of ocean acidification, Nat. Clim. Change, 2013, 3, 975–978 CrossRef CAS.
- D. G. Petersen and I. Dahllöf, Combined effects of pyrene and UV-light on algae and bacteria in an Arctic sediment, Ecotoxicology, 2007, 16, 371–377 CrossRef CAS PubMed.
- C. J. Galbán-Malagón, A. Cabrerizo, N. Berrojálbiz, M. J. Ojeda and J. Dachs, Air-water exchange and phytoplankton accumulation of persistent organic pollutants in the Greenland current and Arctic Ocean, http://132.246.11.198/2012-ipy/pdf-all/ipy2012arAbstract00801.pdf.
- M. Piepho, M. T. Arts and A. Wacker, Species-specific variation in fatty acid concentrations of four phytoplankton species: Does phosphorus supply influence the effect of light intensity or temperature?, J. Phycol., 2012, 48, 64–73 CrossRef CAS.
- D.-P. Häder, P. Richter, V. E. Villafañe and E. W. Helbling, Influence of light history on the photosynthetic and motility responses of Gymnodinium chlorophorum exposed to UVR and different temperatures, J. Photochem. Photobiol., B, 2014 Search PubMed , in press.
- S. Arndt, G. Lacroix, N. Gypens, P. Regnier and C. Lancelot, Nutrient dynamics and phytoplankton development along an estuary–coastal zone continuum: a model study, J. Mar. Syst., 2011, 84, 49–66 CrossRef.
- M. P. Skinner, R. J. Lewis and S. Morton, Ecology of the ciguatera causing dinoflagellates from the Northern Great Barrier Reef: Changes in community distribution and coastal eutrophication, Mar. Pollut. Bull., 2013, 77, 210–219 CrossRef CAS PubMed.
- P. Echeveste, S. Agustí and J. Dachs, Cell size dependence of additive versus synergetic effects of UV radiation and PAHs on oceanic phytoplankton, Environ. Pollut., 2011, 159, 1307–1316 CrossRef CAS PubMed.
- M. H. Olson and N. E. Barbieri, Mechanisms of ultraviolet radiation tolerance in the freshwater snail Physa acuta, Freshwater Sci., 2013, 33, 66–72 CrossRef.
- J. Ahlgren, X. Yang, L.-A. Hansson and C. Brönmark, Camouflaged or tanned: plasticity in freshwater snail pigmentation, Biol. Lett., 2013, 9, 20130464 CrossRef PubMed.
- S. Nahon, A. M. Pruski, J. C. Duchene, L. Mejanelle, G. Vetion, M. Desmalades and F. Charles, Can UV radiation affect benthic deposit-feeders through biochemical alteration of food resources? An experimental study with juveniles of the benthic polychaete Eupolymnia nebulosa, Mar. Environ. Res., 2011, 71, 266–274 CrossRef CAS PubMed.
- L. Van Den Broecke, K. Martens, V. Pieri and I. Schön, Ostracod valves as efficient UV protection, J. Limnol., 2012, 71 Search PubMed.
- K. Bischof and F. S. Steinhoff, Impacts of stratospheric ozone depletion and solar UVB radiation on seaweeds, in Seaweed Biology, Springer, Berlin, Heidelberg, 2012, vol. 219, pp. 433–448 Search PubMed.
- M. Olischläger and C. Wiencke, Seasonal fertility and combined effects of temperature and UV-radiation on Alaria esculenta and Laminaria digitata (Phaeophyceae) from Spitsbergen, Polar Biol., 2013, 36, 1019–1029 CrossRef.
- A. Fricke, M. Molis, C. Wiencke, N. Valdivia and A. S. Chapman, Effects of UV radiation on the structure of Arctic macrobenthic communities, Polar Biol., 2011, 34, 995–1009 CrossRef.
- A. R. Davis, D. Coleman, A. Broad, M. Byrne, S. A. Dworjanyn and R. Przeslawski, Complex responses of intertidal molluscan embroys to a warming and acidifying ocean in the presence of UV radiation, PLoS One, 2013, 8, e55939 CAS.
- C. Vadadi-Fulop, C. Sipkay, G. Meszaros and L. Hufnagel, Climate change and freshwater zooplankton: what does it boil down to?, Aquat. Ecol., 2012, 46, 501–519 CrossRef.
- L. Nevalainen and M. Rautio, Cladoceran carapace absorbance as a new method for inferring past UV radiation exposure of aquatic biota, Quat. Sci. Rev., 2013, 84, 109–115 CrossRef.
- S. Hylander, J. C. Grenvald and T. Kiorboe, Fitness costs and benefits of UVR exposure in marine pelagic copepods, Funct. Ecol., 2014, 28, 149–158 CrossRef.
- C. E. Williamson, J. M. Fischer, S. M. Bollens, E. P. Overholt and J. K. Breckenridge, Toward a more comprehensive theory of zooplankton diel vertical migration: Integrating ultraviolet radiation and water transparency into the biotic paradigm, Limnol. Oceanogr., 2011, 56, 1603–1623 CrossRef.
- R. Tiberti and M. Barbieri, Evidences of zooplankton vertical migration in stocked and never-stocked alpine lakes in Gran Paradiso National Park (Italy), Oceanol. Hydrobiol. Stud., 2011, 40, 36–42 Search PubMed.
- B. Pietrzak, A. Bednarska, M. Markowska, M. Rojek, E. Szymanska and M. Slusarczyk, Behavioural and physiological mechanisms behind extreme longevity in Daphnia, Hydrobiologia, 2013, 715, 125–134 CrossRef CAS.
- R. Tiberti and R. Iacobuzio, Does the fish presence influence the diurnal vertical distribution of zooplankton in high transparency lakes?, Hydrobiologia, 2013, 709, 27–39 CrossRef.
- K. C. Rose, C. E. Williamson, J. M. Fischer, S. J. Connelly, M. Olson, A. J. Tucker and D. A. Noe, The role of UV and fish in regulating the vertical distribution of Daphnia, Limnol. Oceanogr., 2012, 57, 1867–1876 CrossRef.
- D. M. Fields, C. M. F. Durif, R. M. Bjelland, S. D. Shema, A. B. Skiftesvik and H. I. Browman, Grazing rates of Calanus finmarchicus on Thalassiosira weissflogii cultured under different levels of ultraviolet radiation, PLoS One, 2011, 6 Search PubMed.
- M. S. Souza, L. A. Hansson, S. Hylander, B. Modenutti and E. Balseiro, Rapid enzymatic response to compensate UV radiation in copepods, PLoS One, 2012, 7, e32046 CAS.
- S. Hylander, M. S. Souza, E. Balseiro, B. Modenutti and L. A. Hansson, Fish-mediated trait compensation in zooplankton, Funct. Ecol., 2012, 26, 608–615 CrossRef.
- T. Schneider, A. Herzig, K. A. Koinig and R. Sommaruga, Copepods in turbid shallow soda lakes accumulate unexpected high levels of carotenoids, PLoS One, 2012, 7, e43063 CAS.
- A. J. Tucker and C. E. Williamson, The invasion window for warmwater fish in clearwater lakes: the role of ultraviolet radiation and temperature, Div. Distrib., 2014, 20, 181–192 CrossRef.
- A. J. Tucker, C. E. Williamson and J. T. Oris, Development and application of a UV attainment threshold for the prevention of warmwater aquatic invasive species, Biol. Invasions, 2012, 14, 2331–2342 CrossRef.
- A. K. Gevertz, A. J. Tucker, A. M. Bowling, C. E. Williamson and J. T. Oris, Differential tolerance of native and nonnative fish exposed to ultraviolet radiation and fluoranthene in Lake Tahoe (California/Nevada), USA, Environ. Toxicol. Chem., 2012, 31, 1129–1135 CrossRef CAS PubMed.
- A. K. Gevertz and J. T. Oris, Microscopic examination of skin in native and nonnative fish from Lake Tahoe exposed to ultraviolet radiation and fluoranthene, Aquat. Toxicol., 2014, 147, 151–157 CrossRef CAS PubMed.
- V. Boily, A. Bertolo, P. Magnan, M.-G. Martinoli and H.-M. Thérien, The effects of UVR irradiance and spectral composition on yellow perch (Perca flavescens) larvae survival, Aquat. Sci., 2011, 73, 345–354 CrossRef.
- M. C. Melnychuk, C. J. Walters, V. Christensen, M. L. Bothwell and D. W. Welch, Effects of solar ultraviolet radiation exposure on early ocean survival and fry-to-smolt growth of juvenile salmon, Mar. Ecol.: Prog. Ser., 2012, 457, 251–264 CrossRef.
- E. Sucre, F. Vidussi, B. Mostajir, G. Charmantier and C. Lorin-Nebel, Impact of ultraviolet-B radiation on planktonic fish larvae: Alteration of the osmoregulatory function, Aquat. Toxicol., 2012, 109, 194–201 CrossRef CAS PubMed.
- Y. Fukunishi, H. I. Browman, C. M. F. Durif, R. M. Bjelland and A. B. Skiftesvik, Effect of sub-lethal exposure to ultraviolet radiation on the escape performance of atlantic cod larvae (Gadus morhua), PLoS One, 2012, 7, e35554 CAS.
- Y. Fukunishi, H. I. Browman, C. M. F. Durif, R. M. Bjelland, S. D. Shema, D. M. Fields and A. B. Skiftesvik, Sub-lethal exposure to ultraviolet radiation reduces prey consumption by Atlantic cod larvae (Gadus morhua), Mar. Biol., 2013, 160, 2591–2596 CrossRef CAS.
- D. M. Leech, W. J. Boeing, S. L. Cooke, C. E. Williamson and L. Torres, UV-enhanced fish predation and the differential migration of zooplankton in response to UV radiation and fish, Limnol. Oceanogr., 2009, 54, 1152–1161 CrossRef CAS.
- A. G. Finstad, I. P. Helland, O. Ugedal, T. Hesthagen and D. O. Hessen, Unimodal response of fish yield to dissolved organic carbon, Ecol. Lett., 2014, 17, 36–43 CrossRef PubMed.
- J. H. Olker, L. B. Johnson, R. P. Axler and C. M. Johnson, Factors influencing ultraviolet radiation dose to developing frogs in northern vernal pools, Can. J. Fish. Aquat. Sci., 2013, 70, 1531–1541 CrossRef.
- R. Danovaro, L. Bongiorni, C. Corinaldesi, D. Giovannelli, E. Damiani, P. Astolfi, L. Greci and A. Pusceddu, Sunscreens cause coral bleaching by promoting viral infections, Environ. Health Perspect., 2008, 116, 441–447 CAS.
- A. Bowman, L. M. Martinez-Levasseur, K. Acevedo-Whitehouse, D. Gendron and M. A. Birch-Machin, The simultaneous detection of mitochondrial DNA damage from sun-exposed skin of three whale species and its association with UV-induced microscopic lesions and apoptosis, Mitochondrion, 2013, 13, 342–349 CrossRef CAS PubMed.
- L. M. Martinez-Levasseur, M. A. Birch-Machin, A. Bowman, D. Gendron, E. Weatherhead, R. J. Knell and K. Acevedo-Whitehouse, Whales use distinct strategies to counteracts solar ultraviolet radiation, Sci. Rep., 2013, 3, 2386 Search PubMed.
- P. Gago-Ferrero, M. B. Alonso, C. P. Bertozzi, J. Mango, L. Barbosa, M. Cremer, E. R. Secchi, C. Domit, A. Azevedo, J. Lailson-Brito, J. P. M. Torres, O. Malm, E. Eljarrat, M. S. Diaz-Cruz and D. Barcelo, First determination of UVf filters in marine mammals. Octocrylene levels in Franciscana dolphins, Environ. Sci. Technol., 2013, 47, 5619–5625 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry and Owner Societies 2015 |
