Sodium periodate mediated oxidative transformations in organic synthesis
Abstract
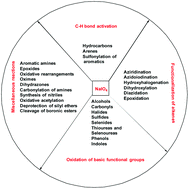
The investigation of new oxidative transformations for the synthesis of carbon–heteroatom and heteroatom–heteroatom bonds is of fundamental importance in the synthesis of numerous bioactive molecules and fine chemicals. In this context, NaIO4, an exciting reagent, has attracted increasing attention enabling the development of these unprecedented oxidative transformations that are difficult to achieve otherwise. Thus, NaIO4 has been successfully explored as a versatile oxidant for a variety of fundamental organic transformations such as C–H activation, oxidative functionalization of alkenes and other interesting oxidative transformations and its application in the synthesis of bioactive natural products. This review summarizes recent developments in this area with NaIO4 as a versatile oxidant and brings out many challenges that still remain elusive for the future.

 Please wait while we load your content...
Please wait while we load your content...