Open Access Article
Open Access ArticleA mechanistic proposal for the protodeboronation of neat boronic acids: boronic acid mediated reaction in the solid state†
Gary
Noonan
*a and
Andrew G.
Leach
 *b
*b
aAstraZeneca, Alderley Park, Macclesfield, SK10 4TG, UK. E-mail: noonangary64@googlemail.com
bSchool of Pharmacy and Biomolecular Sciences, Liverpool John Moores University, James Parsons Building, Byrom Street, Liverpool, L3 3AF, UK. E-mail: a.g.leach@ljmu.ac.uk
First published on 21st January 2015
Abstract
A combined experimental and computational study suggests that a reduction in the entropy of activation in the solid state can lead to the protodeboronation of boronic acids.
Among the ways in which enzymes accelerate bimolecular reactions, one of the least contentious is that they organize the reacting molecules such that they are primed for reaction.1,2 Similar factors contribute to reaction acceleration by catalytic RNA and organic capsules.3 The entropic barrier to the reaction is reduced by the formation of stabilising interactions between the reactants and the catalyst; the reaction is rendered intramolecular. The formation of a crystalline lattice also involves molecular organization and if it arranges functional groups in such a way that they are primed for reaction, an accelerated reaction could result. Estimates of the entropic contribution to the free energy barrier for bimolecular reactions in solution at room temperature range from 2.5 kcal mol−1 to 10.4 kcal mol−1.2,4 Removing even the low end of this range would amount to a large acceleration. As detailed below, we believe such an acceleration, caused by formation of the solid state, contributes to the protodeboronation of boronic acids.
Boronic acids are ubiquitous reagents in modern synthetic organic chemistry, due to their utility in Suzuki–Miyaura cross-coupling (SMCC) and many other synthetic transformations.5–8 Most are stable reagents but certain examples (e.g. 2-heteroarylboronic acids) are susceptible to degradation via protodeboronation (PDeB), either under storage as the neat reagent, or under SMCC conditions.7,9–12 Some ‘protected’ derivatives have been developed to allow long-term storage and slow-release of delicate boronic acids under aqueous basic reaction conditions,9–15 but these reagents are less synthetically efficient and, for some, subtle changes in reaction conditions can affect their rates of release.13–15 By understanding PDeB we wished to enable the use of the more efficient parent boronic acids where possible.
Kuivila and co-workers performed extensive studies of PDeB under aqueous acid and base conditions and revealed mechanistic details of both.16–19 These are unlikely to be relevant here because boronic acids are not strong acids like those shown to be required to promote PDeB.16–19 The neutral pathway proposed by Kuivila also relies upon their acidity, which requires addition of water with liberation of a proton, and is highly unlikely under neutral conditions.18
The first decomposition pathway considered by ourselves was that PDeB of neat boronic acids occurs upon long-term exposure to atmospheric water during storage. Computationally, two mechanisms were found. The first involves one water molecule and a one-step process; the water molecule provides a proton to the aromatic carbon atom while simultaneously forming an O–B bond and cleaving the C–B bond. The transition state for this process, TS1, is shown in Fig. 1 and corresponds to a free energy barrier of 47.0 kcal mol−1. All calculations employed the M06/6-311+G**//B3LYP/6-31+G** level of theory with PCM solvation and were performed in Gaussian09 and 03.20–27 Benchmarking calculations revealed that this level of theory performs well compared to MP2/6-311+G** calculations (see ESI†). Plumley and Evanseck have shown that M06-2X performs better than M06 for borane–ammonia complexes but Lee et al. selected M06 as a more accurate level of theory, in line with our own findings.28–31
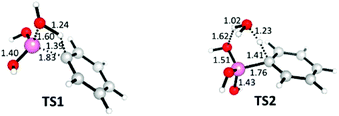 | ||
| Fig. 1 Two transition states for PDeB of phenyl boronic acid by water. Distances given in Å. Boron atoms are shown in pink. | ||
A six-membered ring transition state alternative, in which a second water molecule facilitates the reaction, was also located and the lowest energy possibility is shown as TS2 in Fig. 1. Structure TS2 corresponds to a gas phase enthalpy of activation (Hact) of 19.8 kcal mol−1 that is lower than TS1, which has a computed Hact of 34.0 kcal mol−1. However, the entropic penalty involved in bringing three species together in TS2 results in an aqueous phase free energy barrier that is 3.3 kcal mol−1 higher than that for TS1. Despite the errors inherent to the calculations, these barriers are inconsistent with degradation over the course of days to years. The observation of Hall, that water might actually stabilise boronic acids is, at the least, not impossible.32 The measurements of half lives and activation free energies for a range of biologically important reactions in water by Wolfenden and co-workers (plotted in ESI†) suggest that degradation over the course of days to years would be consistent with free energy barriers in the 27–33 kcal mol−1 range.33
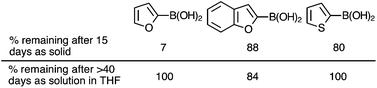
Three examples were selected for experimental study in order to verify this surprising finding (i.e. that water is not the causative agent in PDeB): furan-2-yl-, thiophen-2-yl- and benzofuran-2-yl-boronic acid, each of which have been reported to degrade significantly within 15 days when stored as neat reagents.9 When heated under microwave irradiation to 130 °C for 30 minutes, in either water alone or in tetrahydrofuran![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (4
water (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixtures (to aid solubility of the boronic acid), little or no degradation could be observed when the reaction mixtures were analyzed by LCMS (see ESI†). The lack of decomposition of furan-2-yl boronic acid under these conditions is especially surprising because this is reported to be one of the more unstable heterocyclic boronic acids.9 Experiment and theory concur that water alone is not responsible for the PDeB of boronic acids upon storage.
1) mixtures (to aid solubility of the boronic acid), little or no degradation could be observed when the reaction mixtures were analyzed by LCMS (see ESI†). The lack of decomposition of furan-2-yl boronic acid under these conditions is especially surprising because this is reported to be one of the more unstable heterocyclic boronic acids.9 Experiment and theory concur that water alone is not responsible for the PDeB of boronic acids upon storage.
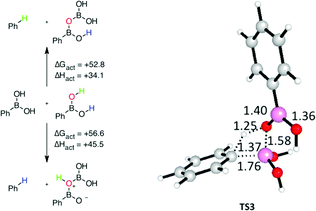
Attention was next turned to the boronic acid molecules themselves. Transition states analogous to TS1 but where a boronic acid provides both the Lewis basic oxygen atom and the requisite proton were computed: the lowest enthalpy example is TS3. The free energy barriers for these processes (Fig. 2) are also prohibitively high, with the lowest being over 50 kcal mol−1. If, however, there were a reduced or eliminated entropic barrier, the enthalpic barrier (34.1 kcal mol−1) would be in line with the free energy barrier for a process that occurs at room temperature over the course of days to years.
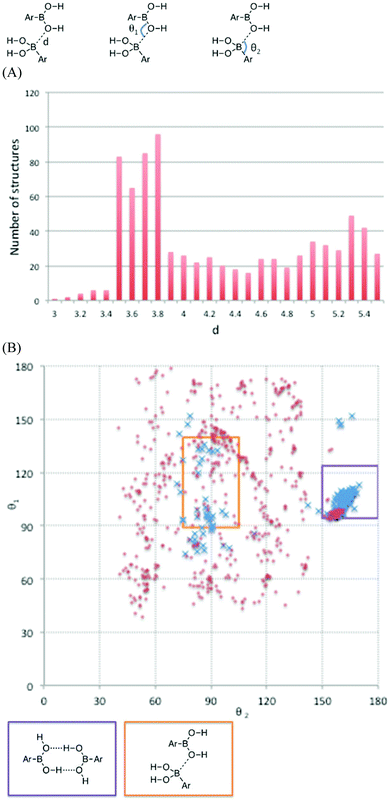
During formation of the solid state, various crystal packing forces determine the arrangement of the functional groups and might pre-organize boronic acids for PDeB, thereby reducing the entropic cost of bringing two molecules together. All of the crystal structures available for boronic acids published in the Cambridge crystallographic database (CCD) were retrieved and several geometrical parameters, describing the position of each boronic acid relative to its neighbors, were measured (Fig. 3).34
The distribution of boron to oxygen distances, d, is shown in Fig. 3(A). The number of structures with d less than 4 Å is enhanced and those with d less than 3.7 Å are shown as blue crosses in Fig. 3(B). The plot of θ1vs. θ2 shown in Fig. 3(B), identifies commonly occurring supramolecular structural features. The area highlighted by the purple box corresponds to the cyclic hydrogen bonding interaction shown below the plot. Hall stated that boronic acids regularly form these interactions in the solid state.32 The orange box in Fig. 3(B) corresponds to an interaction between the oxygen lone pair of one boronic acid and the Lewis-acidic boron atom of another (assuming lone pairs in the 89–140° positions on the oxygen atoms) and represents examples ‘primed for PDeB’. It is found that 16 of the 74 (21.6%) crystal structures that have d < 3.7 Å satisfy these criteria. The crystal structure of phenylboronic acid illustrates the key solid-state structural motifs (end-to-end hydrogen bonding and boron–oxygen interaction) for boronic acids and is shown in Fig. 4.35 Further crystal structure analysis is presented in the ESI.† The manuscripts describing the preparation of material leading to the crystal structures represented by the blue crosses in the orange box were examined. The yield of the compound leading to this crystal structure was compared to that of all other boronic acids described in the same paper.35–54 Such compounds were prepared under similar conditions and so their yields can reasonably be compared. It was found (see ESI†) that compounds which form crystal structures that satisfy the geometric requirements highlighted in Fig. 3 are formed in 11% lower yield on average than other boronic acids reported in the same paper. Given that compounds are presumably prepared in the solid state and studied crystallographically soon after and that the other compounds in the papers remain uncharacterized by crystallography and so may also provide the correct geometries, it is at least suggestive that this geometrically identified subset is prone to faster degradation. Although the pre-organization of boronic acids in the solid state can promote the PDeB reaction, arrangements that place boronic acids in geometries that are not primed for PDeB (the majority) are likely to protect them from degradation. These studies have examined crystalline solids, but the same motifs most likely dominate in amorphous solids which will also include a range of orientations and retain limited mobility.55,56
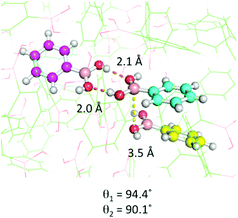 | ||
| Fig. 4 Crystal structure of Ph-B(OH)2. The carbons in three molecules of Ph-B(OH)2 are shown as purple, cyan and yellow spheres while the lattice is shown as green lines. | ||
The consequences of the end-on-end hydrogen bonding interaction were probed by computing energetics in its presence; these are compared to those in its absence in Fig. 5. There is a substantial (9.6 kcal mol−1) driving force for the formation of the hydrogen bond which, although overstated in gas phase calculations, is consistent with the observations shown in Fig. 3. The compromises involved in forming the solid state should ensure that examples which are primed for PDeB have an energy in the region highlighted in purple in Fig. 5, in which the full enthalpy of stabilization due to hydrogen bonding is not achieved and in which the O–B interaction is partially formed. Such a structure is substantially arranged as required for the PDeB process and the enthalpic barrier must therefore be at least 23.2 kcal mol−1 but less than 32.8 kcal mol−1 for phenylboronic acid. Such a value, combined with a modest TΔS component, is entirely consistent with degradation over the course of days to years.
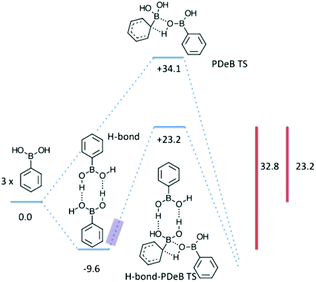 | ||
| Fig. 5 The effect of intermolecular hydrogen bonds on boronic acid mediated PDeB. Gas phase enthalpies in kcal mol−1 relative to three molecules of boronic acid are provided. | ||
The enthalpic barriers have been computed for the processes shown in Fig. 5 for some of the examples found by Burke and co-workers to degrade over the course of 15 days upon benchtop storage (Table 1).9 The observed levels of degradation are consistent with the magnitude of the computed barriers.
| R | % Boronic acid remaining10 | H rel PDeB TS | H rel H-Bond | H rel H-bond- PDeB TS |
|---|---|---|---|---|
| Phenyl | na | 34.1 | −9.6 | 23.2 |
| 2-Furanyl | 7 | 34.8 | −10.0 | 23.8 |
| 2-Benzofuranyl | 88 | 35.9 | −10.1 | 24.8 |
| 2-Thiophenyl | 80 | 31.2 | −8.5 | 21.0 |
| Vinyl | 5 | 34.0 | −9.5 | 23.7 |
| Cyclopropyl | 31 | 33.9 | −9.6 | 23.2 |
This process for PDeB may be further enhanced because the product of the reaction is computed to be more reactive than the reactant. As summarized in Fig. 6, with each reaction, a larger agglomeration of boronic anhydrides could build up which have enhanced Lewis acidity and are more prone to react with a molecule of boronic acid than their precursor. This would suggest that the appropriately structured crystalline material could degrade very rapidly because each step would create a more reactive molecule and be positioned ready to react. This must be set against an unknown effect upon the entropy of activation. Further investigations would be required to investigate whether this computed effect is manifested experimentally. The six-membered boroxine ring is also computed to be more reactive but to be an energetically disfavored species and we were unable to find evidence of them playing a significant role in PDeB of neat boronic acids.
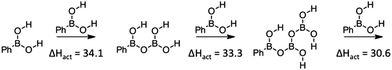 | ||
| Fig. 6 PDeB involving the products formed in boronic acid mediated PDeB. The activation enthalpy for individual steps are provided in kcal mol−1. | ||
The proposed mechanism would imply that unstable boronic acids could be stored in solution. This was found to be the case and boronic acids previously shown to be unstable as neat reagents, over the course of 15 days, undergo little or no decomposition after 1–2 months in THF solution (Fig. 7), even using reagent-grade solvent that is neither dried nor degassed. As well as being of practical relevance to the storage and utility of these reagents, these observations also provide support for a ‘solid-state’ promoted decomposition pathway.
In conclusion, we have carried out a practical and computational study of the possible mechanistic pathways for the PDeB of boronic acids when stored as neat reagents. Prohibitively high computed free-energy barriers for the decomposition of boronic acids by water, together with the practical observation that little PDeB occurs in the presence of excess water suggest that water alone is not the causative agent in PDeB of neat boronic acid samples. We propose a solid-state pre-organization effect, which is backed by the presence of the required supramolecular arrangements in the CCD, as well as by the fact that little or no decomposition is observed for notoriously unstable boronic acids e.g. furan-2-ylboronic acid, when stored in solution for prolonged periods. Computed enthalpies of reaction combined with small entropic barriers, as expected for pre-arranged species, would entail a free energy barrier consistent with the observed rate of degradation. We recommend that unstable boronic acids be stored as solutions whenever they are not required for immediate use.
Acknowledgements
This research was carried out as part of the AstraZeneca global post-doctoral scheme and the authors would like to acknowledge the scientific freedom provided by AstraZeneca throughout the work. Special thanks are due to Dr Jennifer Pink (AstraZeneca) for many useful discussions throughout this research. The authors would also like to acknowledge the use of the EPSRC UK National Service for Computational Chemistry Software (NSCCS) at Imperial College London in carrying out this work.Notes and references
- A. Fersht, in Structure and Mechanism in Protein Science, W. H. Freeman and Co., New York, 1999 Search PubMed.
- M. I. Page and W. P. Jencks, Entropic contributions to rate accelerations in enzymic and intramolecular reactions and the chelate effect, Proc. Natl. Acad. Sci. U. S. A., 1971, 68, 1678–1683 CrossRef CAS.
- S. P. Kim, A. G. Leach and K. N. Houk, The Origins of Noncovalent Catalysis of Intermolecular Diels–Alder Reactions by Cyclodextrins, Self-Assembling Capsules, Antibodies, and RNAses, J. Org. Chem., 2002, 67, 4250–4260 CrossRef CAS PubMed.
- J. Villa, et al., How important are entropic contributions to enzyme catalysis?, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 11899–11904 CrossRef CAS PubMed.
- A. d. Meijere and F. Diederich, in Metal-catalyzed cross-coupling reactions, ed. A. de Meijere and F. Diederich, Wiley-VCH, Weinheim, Chichester, 2004 Search PubMed.
- C. C. Johansson Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Palladium-catalyzed cross-coupling: a historical contextual perspective to the 2010 Nobel Prize, Angew. Chem., Int. Ed., 2012, 51, 5062–5085 CrossRef CAS PubMed.
- D. G. Hall, in Boronic acids: volume 1: preparation and applications in organic synthesis, medicine and materials, Wiley-VCH, Weinheim, 2011 Search PubMed.
- S. Kotha, K. Lahiri and D. Kashinath, Recent applications of the Suzuki–Miyaura cross-coupling reaction in organic synthesis, Tetrahedron, 2002, 58, 9633–9695 CrossRef CAS.
- D. M. Knapp, E. P. Gillis and M. D. Burke, A General Solution for Unstable Boronic Acids: Slow-Release Cross-Coupling from Air-Stable MIDA Boronates, J. Am. Chem. Soc., 2009, 131, 6961–6963 CrossRef CAS PubMed.
- S. Darses and J. P. Genet, Potassium organotrifluoroborates: new perspectives in organic synthesis, Chem. Rev., 2008, 108, 288–325 CrossRef CAS PubMed.
- E. Vedejs, R. Chapman, S. Fields, S. Lin and M. Schrimpf, Conversion of arylboronic acids into potassium aryltrifluoroborates: Convenient precursors of arylboron difluoride lewis acids, J. Org. Chem., 1995, 60, 3020–3027 CrossRef CAS.
- G. A. Molander and B. Canturk, Organotrifluoroborates and monocoordinated palladium complexes as catalysts—a perfect combination for Suzuki–Miyaura coupling, Angew. Chem., Int. Ed., 2009, 48, 9240–9261 CrossRef CAS PubMed.
- M. Butters, et al., Aryl trifluoroborates in Suzuki–Miyaura coupling: the roles of endogenous aryl boronic acid and fluoride, Angew. Chem., Int. Ed., 2010, 49, 5156–5160 CrossRef CAS PubMed.
- A. J. J. Lennox and G. C. Lloyd-Jones, The slow-release strategy in Suzuki-Miyaura coupling, Isr. J. Chem., 2010, 50, 664–674 CrossRef CAS.
- A. J. Lennox and G. C. Lloyd-Jones, Organotrifluoroborate Hydrolysis: Boronic Acid Release Mechanism and an Acid–Base Paradox in Cross-Coupling, J. Am. Chem. Soc., 2012, 134, 7431–7441 CrossRef CAS PubMed.
- H. G. Kuivila and K. V. Nahabedian, Electrophilic Displacement Reactions. XI. Solvent Isotope Effects in the Protodeboronation of Areneboronic Acids1-3, J. Am. Chem. Soc., 1961, 83, 2164–2166 CrossRef CAS.
- H. G. Kuivila and K. V. Nahabedian, Electrophilic Displacement Reactions. X. General Acid Catalysis in the Protodeboronation of Areneboronic Acids1-3, J. Am. Chem. Soc., 1961, 83, 2159–2163 CrossRef CAS.
- H. G. Kuivila, J. F. Reuwer Jr. and J. A. Mangravite, Electrophilic displacement reactions: XV. Kinetics and mechanism of the base-catalyzed protodeboronation of areneboronic acids, Can. J. Chem., 1963, 41, 3081–3090 CrossRef CAS.
- K. V. Nahabedian and H. G. Kuivila, Electrophilic Displacement Reactions. XII. Substituent Effects in the Protodeboronation of Areneboronic Acids1-3, J. Am. Chem. Soc., 1961, 83, 2167–2174 CrossRef CAS.
- Y. Zhao and D. G. Truhlar, The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals, Theor. Chem. Acc., 2008, 120, 215–241 CrossRef CAS.
- Y. Zhao and D. G. Truhlar, Density Functionals with Broad Applicability in Chemistry, Acc. Chem. Res., 2008, 41, 157–167 CrossRef CAS PubMed.
- A. D. Becke, Density-functional thermochemistry. III. The role of exact exchange, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS PubMed.
- C. Lee, W. Yang and R. G. Parr, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density, Phys. Rev. B: Condens. Matter, 1988, 37, 785–789 CrossRef CAS.
- P. C. Hariharan and J. A. Pople, Influence of polarization functions on MO hydrogenation energies, Theor. Chim. Acta, 1973, 28, 213–222 CrossRef CAS.
- J. Tomasi, B. Mennucci and E. Cances, The IEF version of the PCM solvation method: an overview of a new method addressed to study molecular solutes at the QM ab initio level, J. Mol. Struct. (THEOCHEM), 1999, 464, 211–226 CrossRef CAS.
- M. J. Frisch, et al., Gaussian 09 (Revision A.1) Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, Gaussian 03 (Revision C.02), Gaussian, Inc., Wallingford CT, 2004 Search PubMed.
- J. A. Plumley and J. D. Evanseck, Covalent and ionic nature of the dative bond and account of accurate ammonia borane binding enthalpies, J. Phys. Chem. A, 2007, 111, 13472–13483 CrossRef CAS PubMed.
- J. A. Plumley and J. D. Evanseck, Hybrid Meta-Generalized Gradient Functional Modeling of Boron–Nitrogen Coordinate Covalent Bonds, J. Chem. Theory Comput., 2008, 4, 1249–1253 CrossRef CAS.
- J. A. Plumley and J. D. Evanseck, Periodic Trends and Index of Boron Lewis Acidity, J. Phys. Chem. A, 2009, 113, 5985–5992 CrossRef CAS PubMed.
- J. M. Lee, P. Helquist and O. Wiest, Diastereoselectivity in Lewis-Acid-Catalyzed Mukaiyama Aldol Reactions: A DFT Study, J. Am. Chem. Soc., 2012, 134, 14973–14981 CrossRef CAS PubMed.
- D. G. Hall, in Boronic acids: preparation and applications in organic synthesis and medicine, Wiley-VCH, John Wiley, Weinheim, Chichester, 2005 Search PubMed.
- R. Wolfenden, Benchmark reaction rates, the stability of biological molecules in water, and the evolution of catalytic power in enzymes, Annu. Rev. Biochem., 2011, 80, 645–667 CrossRef CAS PubMed.
- F. H. Allen, The Cambridge Structural Database: a quarter of a million crystal structures and rising, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 380–388 CrossRef PubMed.
- S. J. Rettig and J. Trotter, Crystal and molecular structure of phenylboronic acid, C6H5B(OH)2, Can. J. Chem., 1977, 55, 3071–3075 CAS.
- C. Zheng, B. Spielvogel, R. Smith and N. Hosmane, Crystal structure of 4-methylphenylboronic acid, C7H9BO2, Z. Kristallogr. - New Cryst. Struct., 2001, 216, 363–364 Search PubMed.
- N. Saygili, A. S. Batsanov and M. R. Bryce, 5-Pyrimidylboronic acid and 2-methoxy-5-pyrimidylboronic acid: new heteroarylpyrimidine derivatives via Suzuki cross-coupling reactions, Org. Biomol. Chem., 2004, 2, 852–857 CAS.
- P. Rodríguez-Cuamatzi, G. Vargas-Díaz and H. Höpfl, Modification of 2D water that contains hexameric units in chair and boat conformations—a contribution to the structural elucidation of bulk water, Angew. Chem., Int. Ed., 2004, 116, 3103–3106 CrossRef.
- K. M. Clapham, et al., New Pyrimidylboronic Acids and Functionalized Heteroarylpyrimidines by Suzuki Cross-Coupling Reactions, Eur. J. Org. Chem., 2007, 5712–5716 CrossRef CAS.
- A. E. Smith, K. M. Clapham, A. S. Batsanov, M. R. Bryce and B. Tarbit, (Dimethoxy-and Dihalopyridyl) boronic Acids and Highly Functionalized Heteroarylpyridines by Suzuki Cross-Coupling Reactions, Eur. J. Org. Chem., 2008, 1458–1463 CrossRef CAS.
- K. M. Clapham, A. S. Batsanov, M. R. Bryce and B. Tarbit, Trifluoromethyl-substituted pyridyl- and pyrazolylboronic acids and esters: synthesis and Suzuki–Miyaura cross-coupling reactions, Org. Biomol. Chem., 2009, 7, 2155–2161 CAS.
- M. Dabrowski, S. Lulinski, J. Serwatowski and M. Szczerbinska, (2-Methoxy-3-pyridyl) boronic acid, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2006, 62, o702–o704 CAS.
- K. Kacprzak, T. Klis and J. Serwatowski, [3-Bromo-2-(3-fluorobenzyloxy) phenyl] boronic acid, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2009, 65, o2250–o2250 CAS.
- M. R. Shimpi, N. SeethaLekshmi and V. R. Pedireddi, Supramolecular architecture in some 4-halophenylboronic acids, Cryst. Growth Des., 2007, 7, 1958–1963 CAS.
- S. Das, V. L. Alexeev, A. C. Sharma, S. J. Geib and S. A. Asher, Synthesis and crystal structure of 4-amino-3-fluorophenylboronic acid, Tetrahedron Lett., 2003, 44, 7719–7722 CrossRef CAS PubMed.
- C. B. Aakeröy, J. Desper, B. Levin and D. J. Salmon, Boronic Acids As Versatile Supramolecular Reagents, ACA Trans., 2004, 39, 123–129 Search PubMed.
- M. K. Cyrański, A. Jezierska, P. Klimentowska, J. J. Panek and A. Sporzyński, Impact of intermolecular hydrogen bond on structural properties of phenylboronic acid: quantum chemical and X-ray study, J. Phys. Org. Chem., 2008, 21, 472–482 CrossRef.
- R. M. Al-Zoubi and D. G. Hall, Mild silver (I)-mediated regioselective iodination and bromination of arylboronic acids, Org. Lett., 2010, 12, 2480–2483 CrossRef CAS PubMed.
- J. Serwatowski, T. Klis and K. Kacprzak, 3-Bromo-2-(2-fluorobenzyloxy) phenylboronic acid, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2006, 62, o1308–o1309 CAS.
- R. Sarma and J. B. Baruah, B⋯π-aromatic and C–H⋯B interactions in co-crystals of aromatic amine N-oxides with p-phenylenediboronic acid, J. Mol. Struct., 2009, 920, 350–354 CrossRef CAS PubMed.
- A. E. Thompson, et al., 2-Ethoxy-3-pyridylboronic acid: a versatile reagent for the synthesis of highly-functionalised 3-aryl/heteroaryl-pyridines via Suzuki cross-coupling reactions, Tetrahedron, 2005, 61, 5131–5135 CrossRef CAS PubMed.
- S. Lulinski and J. Serwatowski, 2-(Methoxycarbonyl) phenylboronic acid, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2006, 62, o301–o303 Search PubMed.
- M. Filthaus, I. M. Oppel and H. F. Bettinger, Supramolecular structures and spontaneous resolution: the case of ortho-substituted phenylboronic acids, Org. Biomol. Chem., 2008, 6, 1201–1207 CAS.
- M. Dabrowski, S. Lulinski and J. Serwatowski, (2-Methoxy-1, 3-phenylene) diboronic acid, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2008, 64, o414–o415 CAS.
- L. Yu, Amorphous pharmaceutical solids: preparation, characterization and stabilization, Adv. Drug Delivery Rev., 2001, 48, 27–42 CrossRef CAS.
- M. C. Wilding, M. Wilson and P. F. McMillan, Structural studies and polymorphism in amorphous solids and liquids at high pressure, Chem. Soc. Rev., 2006, 35, 964–986 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: All experimental and analytical information for compounds described, stability studies, computed geometries and energies, computational benchmarking, solid state parameters. See DOI: 10.1039/c4ob02543a |
| This journal is © The Royal Society of Chemistry 2015 |