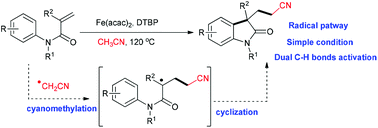
Fe-promoted radical cyanomethylation/arylation of arylacrylamides to access oxindoles via cleavage of the sp3 C–H of acetonitrile and the sp2 C–H of the phenyl group†
Abstract
Radical cyanomethylation/arylation of arylacrylamides to access oxindoles with acetonitrile as the radical precursor is described. This reaction involves dual C–H bond functionalization, including the sp3 C–H of acetonitrile and the sp2 C–H of the phenyl group. A variety of functional groups, such as methoxy, ethyloxy carbonyl, chloro, bromo, iodo, nitro, trifluoromethoxy and trifluoromethyl groups, are well tolerated.

 Please wait while we load your content...
Please wait while we load your content...