One-pot quadruple/triple reaction sequence: a useful tool for the synthesis of natural products
K.
Kashinath
and
D.
Srinivasa Reddy
*
Division of Organic Chemistry, National Chemical Laboratory, Pune, Maharashtra, India. E-mail: ds.reddy@ncl.res.in
First published on 30th October 2014
Abstract
Multiple reactions in one pot has always been a useful technique for synthetic organic chemists, as it can minimizes solvent usage, time and the number of purification steps when compared to individual multi-step syntheses. In line with this, here in this perspective we discuss a one-pot quadruple/triple reaction sequence comprising an enyne ring-closing metathesis/cross-metathesis/Diels–Alder/aromatization for the synthesis of natural products setting.
Introduction
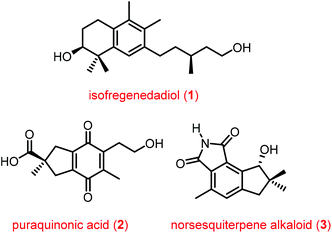
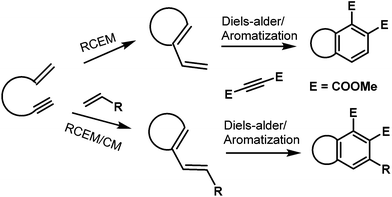
One-pot reactions are a set of reactions where a sequence of chemical transformations are performed in the same reaction flask. They are broadly classified into two categories: the first one refers to domino/cascade reactions in which the starting substrates are designed to undergo several reactions without additional reagents or catalysts. The second category refers to performing multiple reactions in a one-pot process in which additional reagents and catalysts are added at different times. The first kind is extensively studied and has been reviewed several times in the literature.1 The second category is underexplored and recently started gaining momentum.2 The concept “pot economy” was introduced by Clarke et al.,2c which refers to the completion of as many sequential synthetic transformations as possible in the same reaction pot without the need for work-up and product isolation between successive steps. This effort can dramatically reduce the amount of solvent used in reactions, in work-up during the product isolation and purification of intermediate products. Additional benefits are reduction of various materials used in chromatographic purification, contaminated aqueous waste generated while cleaning glassware, time required to do multiple operations etc. Recently, we and others have demonstrated the use of a one-pot quadruple/triple reaction sequence comprising enyne ring-closing metathesis/cross-metathesis/Diels–Alder/aromatization reactions during the total synthesis of natural products.3–5 The metathesis and Diels–Alder reactions are well explored reactions in organic synthesis to form a carbon–carbon bond. The combination of these reactions were elegantly used by several groups to form different molecular frameworks either in a stepwise manner or in a one-pot process.3–7 Although, the combination of these reactions were used for the synthesis of different molecular architectures, their use in synthesis of natural products is very limited. In this perspective, we discuss the synthesis of three biologically interesting natural products isofregenedadiol 1,3 puraquinonic acid 24 and a norsesquiterpene alkaloid 35 (Fig. 1) using the one-pot quadruple/triple reaction sequence. This effort needs to be highlighted so as to encourage the use of greener practices in total synthesis of natural products.A common strategy was used to synthesize the main core of all three natural products listed above and the strategy used for the synthesis is depicted in forward synthetic manner (Fig. 2). An enyne can undergo ring closing enyne metathesis (RCEM) and/or cross metathesis (CM) to afford a diene which can be reacted with a dienophile followed by oxidation to furnish the aromatic skeleton (Fig. 2).
Total synthesis of isofregenedadiol
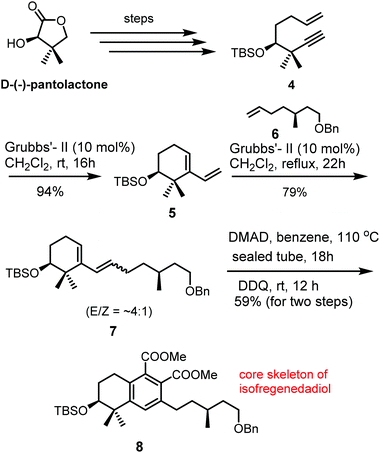
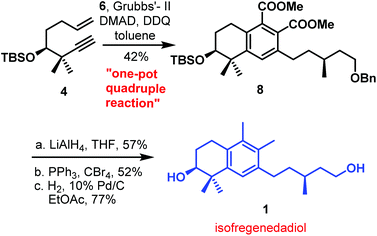
The first total synthesis of biogenetically interesting isofregenedadiol 1, a bicyclic diterpene, was reported by our group in 2011.3 The synthesis commenced from the commercially available D-(−)-pantolactone, a favourite chiral building block for this group.8 The D-(−)-pantolactone was converted into the desired enyne 4 by standard functional group transformations. Initially, we synthesized the desired tetrahydronapthalene skeleton in a sequential manner as shown in Scheme 1. Enyne 4 in the presence of Grubbs’ second generation catalyst, underwent RCEM to produce diene 5 in 94% yield. Compound 5 on cross metathesis with olefin 6 afforded diene 7 in 79% yield, which on Diels–Alder reaction with dimethyl acetylenedicarboxylate (DMAD), followed by aromatization using DDQ, furnished the desired tetrahydronaphthalene derivative 8 in 59% yield for two steps. Having established the route in a sequential manner, we attempted the planned key one-pot quadruple reaction sequence. After a few trials, the planned one-pot sequence proceeded smoothly to furnish the desired tetrahydronaphthalene derivative 8 in 42% overall yield (Scheme 2). The reaction times were chosen for the addition of subsequent reagents and catalysts based on thin-layer chromatography (tlc) monitoring at each step. Although, the obtained yield was moderate, it was very similar (44% vs. 42%) to the overall yield obtained in 4 steps. By this process we could save time, solvent, materials required for chromatography at each step etc. Both carboxylic esters present in compound 8 were transformed to methyl groups and ultimately to the natural product isofregenedadiol 1 with the help of three additional steps (LiAlH4 reduction, diol to dibromide and hydrogenation).Total synthesis of puraquinonic acid
The same one-pot quadruple reaction sequence strategy was used by Gleason et al. in the total synthesis of anti-cancer natural product puraquinonic acid in 2013.4 The synthesis started from the conversion of 9 to enyne 10 in five steps. Initially they attempted ring-closing enyne/diene–ene cross metathesis of 10 with 3-buten-1-ol using Grubbs first/second-generation catalyst, but this afforded only intramolecular enyne metathesis product and the homodimer of 3-buten-1-ol with no desired cross-metathesis products. However, replacement of 3-buten-1-ol with its benzoate ester 11 under the same metathesis conditions, afforded the desired cross metathesis product 12 in 89% yield as a single E isomer. Compound 12 upon Diels–Alder reaction with DMAD followed by DDQ oxidation furnished the desired indane skeleton 13 in 87% yield. After having 13 in hand, they attempted the one-pot quadruple reaction sequence, where they performed the enyne/cross metathesis in dichloromethane, followed by switching the solvent to toluene for the Diels–Alder reaction and DDQ oxidation to afford the densely functionalized indane 13 in 83% yield. Compound 13 was used for the completion of the total synthesis of natural product puraquinonic acid (Scheme 3).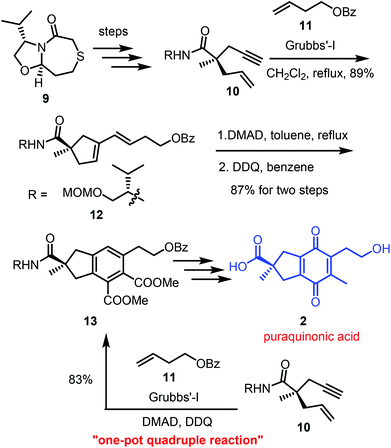 | ||
| Scheme 3 Utilization of one-pot quadruple reaction strategy in total synthesis of puraquinonic acid. | ||
Total synthesis of norsesquiterpene alkaloid
Very recently, the first total synthesis of an anticancer norsesquiterpene alkaloid (R)-8-hydroxy-4,7,7-trimethyl-7,8-dihydrocyclopenta[e]isoindole-1,3(2H,6H)-dione, in both racemic and enantiomeric pure forms, was accomplished by our group.5 The synthesis features a one-pot, three-step reaction sequence comprising an enyne RCM/Diels–Alder/aromatization sequence to construct the desired indane skeleton present in the natural product 3 (Scheme 4). The synthesis began with the preparation of key enyne intermediate 14 from D-(−)-pantolactone. Enyne 14 upon RCM using Grubbs’ 1st generation catalyst followed by Diels–Alder reaction with dimethyl acetylenedicarboxylate (DMAD) and subsequent treatment with DDQ, provided the aromatized compound 15 in moderate yield (∼40%). The indane derivative 15 on ester hydrolysis, heating with urea in ethylene glycol, followed by benzyl deprotection furnished the norsesquiterpene alkaloid.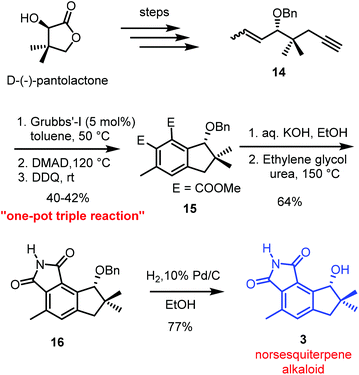 | ||
| Scheme 4 Enantiospecific total synthesis of a norsesquiterpene alkaloid using a one-pot triple reaction strategy. | ||
Conclusion
Organic synthesis is continuously evolving to cater the needs of a changing society. One of such advancements in that direction is implementing green chemistry practices wherever possible during the course of target molecule synthesis. This effort led to an increase in the use of tandem reactions, which combine multiple reactions in a one-pot process, for the synthesis of complex molecules, in particular, in total synthesis of natural products. In that direction, recently, our own research group was able to demonstrate the use of a one-pot quadruple/triple reaction sequence comprising of enyne ring-closing metathesis/cross-metathesis/Diels–Alder/aromatization for the synthesis of natural products like isofregenedadiol and a norsesquiterpene alkaloid. The same one-pot quadruple reaction sequence strategy was used by Gleason et al. in their synthesis of puraquinonic acid. This clearly suggests that the one-pot process that combines several reactions is feasible in the synthesis of natural products. We do understand that there are some limitations where we can only combine certain types of reactions. As we are all striving to make better processes for a better society, more efforts in this direction will certainly overcome these limitations by altering reaction conditions, by choosing appropriate solvents/reagents/ catalysts, by using appropriate devices, etc. At this stage, these efforts may look small and only incremental, we are confident that in the future these practices will have significant impact and efforts in this direction will be appreciated and rewarded.Acknowledgements
We would like to thank all our co-workers who were involved in developing the described research, particularly Mr Suresh Kurhade (Advinus Therapeutics Ltd, Pune). We acknowledge CSIR, New Delhi, for the support through XII Five Year Plan (CSC0130, NAPAHA; BSC0104, SPLENDID). KK also thanks CSIR, New Delhi for the award of a senior research fellowship.Notes and references
- Selected reviews on cascade reactions: (a) C. M. R. Volla, I. Atodiresei and M. Rueping, Chem. Rev., 2014, 114, 2390 CrossRef CAS PubMed; (b) D. B. Ramachary and S. Jain, Org. Biomol. Chem., 2011, 9, 1277 RSC; (c) Ł. Albrecht, H. Jiang and K. A. Jørgensen, Angew. Chem., Int. Ed., 2011, 50, 8492 CrossRef PubMed; (d) C. Grondal, M. Jeanty and D. Enders, Nat. Chem., 2010, 2, 167 CrossRef CAS PubMed; (e) K. C. Nicolaou, D. J. Edmonds and P. G. Bulger, Angew. Chem., Int. Ed., 2006, 45, 7134 CrossRef CAS PubMed.
- Selected references for one-pot reactions: (a) E. D. D. Calder, M. W. Grafton and A. Sutherland, Synlett, 2014, 1068 Search PubMed; (b) C. Vaxelaire, P. Winter and M. Christmann, Angew. Chem., Int. Ed., 2011, 50, 3605 CrossRef CAS PubMed; (c) P. A. Clarke, S. Santos and W. H. C. Martin, Green Chem., 2007, 9, 438 RSC.
- S. E. Kurhade, A. I. Sanchawala, V. Ravikumar, D. Bhuniya and D. S. Reddy, Org. Lett., 2011, 13, 3690 CrossRef CAS PubMed.
- E. A. Tiong, D. Rivalti, B. M. Williams and J. L. Gleason, Angew. Chem., Int. Ed., 2013, 52, 3442 CrossRef CAS PubMed.
- K. Kashinath, P. D. Jadhav and D. S. Reddy, Org. Biomol. Chem., 2014, 12, 4098 CAS.
- Selected references for tandem enyne metathesis/cross metathesis/Diels–Alder reactions: (a) H. Park, Y.-L. Hong, Y. B. Kim and T.-L. Choi, Org. Lett., 2010, 12, 3442 CrossRef CAS PubMed; (b) H.-Y. Lee, H. Y. Kim, H. Tae, B. G. Kim and J. Lee, Org. Lett., 2003, 5, 3439 CrossRef CAS PubMed; (c) S. Kotha, S. Halder, E. Brahmachary and T. Ganesh, Synlett, 2000, 853 CAS.
- Selected references for tandem enyne metathesis/Diels–Alder reactions: (a) S. Kotha, D. Goyal and A. S. Chavan, J. Org. Chem., 2013, 78, 12288 CrossRef CAS PubMed; (b) M. W. Grafton, L. J. Farrugia and A. Sutherland, J. Org. Chem., 2013, 78, 7199 CrossRef CAS PubMed; (c) A. V. Subrahmanyam, K. Palanichamy and K. P. Kaliappan, Chem. – Eur. J., 2010, 16, 8545 CrossRef CAS PubMed; (d) S. Kotha, M. Meshram and A. Tiwari, Chem. Soc. Rev., 2009, 38, 2065 RSC; (e) R. Ben-Othman, M. Othman, S. Coste and B. Decroix, Tetrahedron, 2008, 64, 559 CrossRef CAS PubMed; (f) L. Evanno, A. Deville, B. Bodo and B. Nay, Tetrahedron Lett., 2007, 48, 4331 CrossRef CAS PubMed; (g) K. C. Majumdar, H. Rahaman, S. Muhuri and B. Roy, Synlett, 2006, 466 CrossRef CAS PubMed; (h) M. Rosillo, G. Domínguez, L. Casarrubios, U. Amador and J. Pérez-Castells, J. Org. Chem., 2004, 69, 2084 CrossRef CAS PubMed; (i) Y.-K. Yang and J. Tae, Synlett, 2003, 2017 CAS; (j) S. Kotha, N. Sreenivasachary and E. Brahmachary, Eur. J. Org. Chem., 2001, 787 CrossRef CAS; (k) D. Bentz and S. Laschat, Synthesis, 2000, 1766 CrossRef CAS PubMed.
- See selected previous publications from this group where D-(−)-pantolactone chiral pool was used in the total synthesis of natural products: (a) B. M. Rajesh, M. V. Shinde, M. Kannan, G. Srinivas, J. Iqbal and D. S. Reddy, RSC Adv., 2013, 3, 20291 RSC; (b) K. Kashinath, P. S. Swaroop and D. S. Reddy, RSC Adv., 2012, 2, 3596 RSC; (c) A. K. Hajare, V. Ravikumar, S. Khaleel, D. Bhuniya and D. S. Reddy, J. Org. Chem., 2011, 76, 963 CrossRef CAS PubMed; (d) A. K. Hajare, L. S. Datrange, S. Vyas, D. Bhuniya and D. S. Reddy, Tetrahedron Lett., 2010, 51, 5291 CrossRef CAS PubMed; (e) D. S. Reddy, G. Srinivas, B. M. Rajesh, M. Kannan, T. V. Rajale and J. Iqbal, Tetrahedron Lett., 2006, 47, 6373 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |