Open Access Article
Open Access ArticleTwo-colour fluorescent imaging in organisms using self-assembled nano-systems of upconverting nanoparticles and molecular switches†
Tuoqi
Wu
a,
Bob
Johnsen
b,
Zhaozhao
Qin
b,
Masakazu
Morimoto
c,
David
Baillie
*b,
Masahiro
Irie
*c and
Neil R.
Branda
*a
a4D LABS, Department of Chemistry, Simon Fraser University, 8888 University Drive, Burnaby, BC, Canada V5A 1S6. E-mail: nbranda@sfu.ca; Fax: +1 778 782-3765; Tel: +1 778 782-8051
bDepartment of Molecular Biology and Biochemistry, Simon Fraser University, 8888 University Drive, Burnaby, BC, Canada V5A 1S6
cDepartment of Chemistry and Research Center for Smart Molecules, Rikkyo University, 3-34-1 Nishi-Ikebukuro, Toshima-ku, Tokyo 171-8501, Japan
First published on 3rd June 2015
Abstract
The water-insolubility of a potentially versatile photoresponsive ‘turn-on’ fluorescence probe was overcome by incorporating it into a nano-assembly containing an upconverting nanoparticle wrapped in an amphiphilic polymer. The appeal of the nano-system is not only in the ability to turn “on” and “off” the fluorescence from the organic chromophore using UV and visible light, it is in the fact that the nanoparticle acts as a static probe because it emits red and green light when excited by near infrared light, which is not effected by UV and visible light. This dual-functioning emission behaviour was demonstrated in live organisms.
Introduction
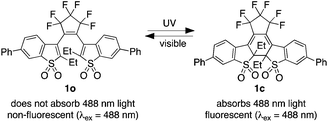
Although the use of conventional fluorescent probes1 for bioimaging applications has proven experimentally and clinically beneficial, the use of ‘turn-on’ fluorescent probes2 has its own merits. These probes are engineered to emit read-out signals only after they are activated by an external stimulus.2 When the external stimulus involves a chemical reaction or interaction, the appearance of emission is a convenient way to validate site-specific binding or detect biological events in cells or living organisms. On the other hand, photoresponsive probes that are turned ‘on’ and ‘off’ using two colours of stimulating light when and where the user desires adds the component of time to imaging methods. This benefit offers the ability to separate the spatially indistinguishable fluorophores temporally, and allows the reconstruction of images with subdiffraction resolution to improve the quality of images.3 These features explain why ‘turn-on’ fluorescent probes are frequently used as tools for cell imaging where they can detect chemical species4 and imaging proteins in cells,5 while photoactive ‘turn-on’ fluorescent probes can be used for super-resolution imaging.3A recently reported example of a probe that changes from a dark, non-emissive state to a bright, emissive state was based on the reversible photochemical reaction of the disulfone 1o shown in Scheme 1.6 This appealing system has an impressive emission contrast ratio between its two states and is based on the well known ring-closing and ring-opening reactions of diarylethenes using two different colours of light.7 In the case of this diarylethene, its non-emissive ring-open isomer 1o is quantitatively converted to its highly emissive ring-closed isomer 1c using UV light (1o → 1c). The broad emission of 1c is between 500 nm and 650 nm when using light of 488 nm as the excitation source. The emission can be turned off using visible light, which triggers the ring-opening reaction (1c → 1o) and regenerates the original non-emissive compound.
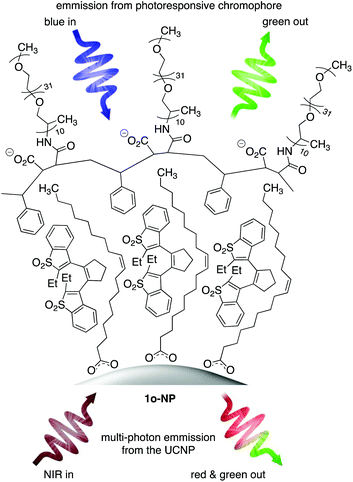
We have demonstrated how multi-component, water-soluble nano-systems are self-assembled when an amphiphilic polymer wraps itself around inorganic upconverting nanoparticles (UCNPs).8 The amphiphilic nature of the polymer ensures the entire nano-system has good compatibility with aqueous environments while providing a lipophilic layer close to the surface of the nanoparticles. It is within this lipophilic layer that hydrophobic compounds such as 1o are entrapped if they are present during the self-assembly process.
In this article, we describe the ‘turn-on’ properties of a self-assembled nano-system containing compound 1o, which becomes reversibly emissive when exposed to UV and visible light. The appeal of the system is that the nanoparticles display their own emission when they absorb several photons of near-infrared (NIR) light and convert them into red and green ones.9 The resulting system is multi-modal (it has two fluorescent probes). The emission from the UCNPs (stimulated by NIR light) is always ‘on’ and can always be used as a read-out signal even when the signal from the ‘turn-on’ probe is ‘off’. Using UV light to trigger the ring-closing reaction of 1o and consequently turning the emission from the organic chromophore ‘on’ provides a greater level of reliability and a reduction in false positive signals when tracking biochemical events.10 We demonstrate the use of our nano-system imaging agent in live Caenorhabditis elegans nematodes.
Results and discussion
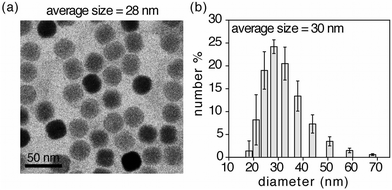
The self-assembled nano-system 1o-NP is shown in Fig. 1 and was synthesized using the reported one-pot protocol8d by treating a CHCl3 dispersion of oleate-coated lanthanide-doped (2 mol% Er3+ and 20% Yb3+) upconverting nanoparticles (NaYF4:ErYb) and poly(styrene-co-maleic anhydride) with poly(propylene glycol)bis(2-aminopropyl ether) to ring-open the anhydride groups. Introducing the hydrophobic chromophore (1o) and replacing the organic solvent with basic water promotes the self-assembly process and produces the nano-system 1o-NP, which were isolated by passing the aqueous sample through syringe filters.‡Transmission electron microscopy (TEM) images of 1o-NP (Fig. 2a) show an average diameter of 28 nm for the nano-systems (Fig. S1†). The average size is measured to be slightly larger (30 nm) by dynamic light scattering (DLS) because this technique measures the average hydrodynamic radius, which includes the polymer shell (Fig. 2b). This organic shell cannot be directly observed by TEM due to its lack of contrast on the TEM grid. The shell thickness of 2 nm is substantially smaller than what it would be if all the polyethylene glycol chains were fully extended (11 nm) suggesting the polymer shell exists in a compressed form as has been previously observed.8a,b
As is typically done for photochromic compounds, the ring-closing (1o-NP → 1c-NP) and ring-opening (1c-NP → 1o-NP) reactions of the chromophores entrapped within the nano-assemblies are best monitored using UV-vis absorption spectroscopy. When an aqueous dispersion of 1o-NP is exposed to 365 nm light,§ the characteristic bands in the visible region of the spectrum (centered at 460 nm) corresponding to the ring-closed isomer 1c appear (Fig. 3a). These bands overlap with those for the ring-closed isomer of the free chromophore in CH3CN. Due to the scattering of the nano-systems in the UV-vis region of the spectrum, the decrease in the higher-energy bands (250 nm to 300 nm) that are characteristic of the ring-closing reaction of diarylethenes (see the spectra of 1o and 1c in Fig. 3a) cannot be observed. In either case, the appearance of the visible bands explains the change in colour of both solutions from colourless to yellow. The spectral changes stop within a minute (30 s for 1o-NP in water and 60 s for 1o in CH3CN) at which time the chromophores have reached their photostationary states. While the amount of ring-closed isomer in the photostationary state of the nano-assmblies (1c-NP) could not be directly measured, it is safe to assume the behaviour is similar to that of the free chromophores,8b,d which ring-close to generate 1c quantitatively as measured by 1H NMR spectroscopy. Based on this assumption, the average loading in 1o-NP was calculated to be 65 chromophores per nanoparticle, which is in accordance with the number of organic molecules encapsulated in the previous reported nano-assemblies synthesized by similar methods.8a,b,d When either coloured solution (1c-NP in water and 1o in CH3CN) is exposed to visible light of wavelengths greater than 434 nm,¶ it reverts back to its colourless state as the ring-open isomers are regenerated. This ring-closing/ring-opening cycle can be repeated without major changes in the spectra (Fig. S6†).
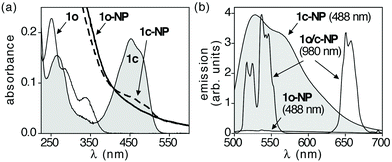 | ||
| Fig. 3 (a) UV-vis absorption spectra of a CH3CN solution (1 × 10−5 M) of chromophore 1o before (white shaded area) and after exposure to 365 nm light (grey shaded area), and an aqueous dispersion (0.8 × 10−6 M)|| of the nano-assemblies (1o-NP) before (solid line) and after exposure to 365 nm light (broken line). (b) Emission spectra for an aqueous dispersion of the nano-assemblies (0.8 × 10−6 M)|| when excited at 488 nm and 980 nm before (1o-NP) and after exposure to 365 nm light (1c-NP). Because the emission from the UCNPs are not affected by the states of the organic chromophores, the two spectra generated with NIR light (1o/c-NP) are shown as superimpositions. The concentrations reported here are concentrations of 1o in free solution or in encapsulated nano-assemblies. | ||
The emission behavior of the nano-assemblies is illustrated in Fig. 3b. When the aqueous dispersion of 1o-NP is excited with 980 nm light, it generates green emission (also shown in photographs in Fig. S4 and S5†). This emission is attributed solely to the UCNPs since neither chromophore 1o nor 1c absorbs NIR light, and exposing a CH3CN solution of 1c produces no observable fluorescence. As is typical for NaYF4:ErYb/NaYF4 nanoparticles, two sets of relatively sharp bands are observed in the region between 500 and 700 nm (Fig. 3b). The higher energy green emission (504–568 nm) correspond to the [2H11/2, 4S3/2] → 4I15/2 transitions, while the lower energy red emission (627–684 nm) are a result of the 4F9/2 → 4I15/2 transitions.
When light at 488 nm is used as excitation source, no fluorescence is observed from the aqueous dispersion of 1o-NP as expected since neither component (1o or the UCNPs) absorbs at 488 nm (Fig. 3b). After exposure to 365 nm light to trigger the ring-closing reaction (1o-NP → 1c-NP) an intense, broad emission band between 500 and 700 nm is observed when the sample is excited at 488 nm. This behavior parallels that of the free chromophore in CH3CN (Fig. S3†). The emission of 1c-NP when it is excited with 980 nm light was almost identical to that of 1o-NP (Fig. S3†), indicating the emission from UCNPs in the nano-assemblies are not effected by the states of surrounding diarylethene molecules. The emission from the organic chromophores could be turned ‘off’ again by exposing the aqueous dispersion to light at wavelengths greater than 480 nm to regenerate the ring-open isomer (1o-NP).
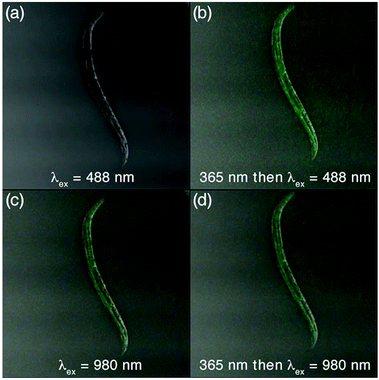
The use of the nano-systems as both conventional and ‘turn-on’ fluorescent probes was demonstrated using Caenorhabditis elegan nematodes L4 hermaphrodites, which were incubated for 3 h with 1o-NP in NaCl buffer.‡ Importantly, the nematodes were still viable after the treatment and incubation. After immobilizing the nematodes with NaN3 in NaCl buffer, they were washed and isolated by centrifugation, then mounted on agarose gel pads and imaged by using 2-photon fluorescence microscopy. The results are shown in the images in Fig. 4.
As anticipated, no fluorescence was observed when the animals are exposed to 488 nm light. However, the emission resulting from the upconverting process of NIR light by the UCNPs in 1o-NP could be clearly observed in the digestive tract of the nematodes, indicating that they had ingested the nanoparticles. Exposing the animals to 365 light for 30 s results in clearly observable emission when excited at 488 nm due to the ring-closing reaction already demonstrated. Once again, the emission from the UCNPs was not effected. Importantly, the entire process caused no observable damage to the nematodes, and the nematodes were still mobile before, during, and after the imaging experiments.**
Conclusions
In this article, we show how we have successfully used water-dispersible nano-assemblies containing photoresponsive diarylethene chromophores and upconverting nanoparticles as dual-functioning fluorescent probes for live organism imaging. The NIR-generated emission from the UCNPs can be used as one (static) probe as it does not change. The second emission can be modulated by toggling the photoresponsive compound between its dark (1o) and bright (1c) state using UV and visible light. Systems such as this not only have the capacity to enhance the quality of images through ‘turn-on’ mechanism,2 they can also reduce false positive signals due to the presence of a second probe.Acknowledgements
This research was supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada and the Canada Research Chairs Program. This work made use of 4D LABS shared facilities supported by the Canada Foundation for Innovation (CFI), British Columbia Knowledge Development Fund (BCKDF) and Simon Fraser University. We thank Dr Saeid Kamal for the help with two-photon microscopy work.Notes and references
- R. Weissleder and M. J. Pittet, Nature, 2008, 452, 580 CrossRef CAS PubMed.
- H. Kobayashi and P. L. Choyke, Acc. Chem. Res., 2011, 44, 83 CrossRef CAS PubMed.
- (a) H. Bo, M. Bates and X. Zhuang, Annu. Rev. Biochem., 2009, 78, 993 CrossRef PubMed; (b) M. Fernandez-Suarez and A. Y. Ting, Nat. Rev., 2008, 9, 929 CrossRef CAS PubMed; (c) F. M. Raymo, Phys. Chem. Chem. Phys., 2013, 15, 14840 RSC.
- (a) X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910 CrossRef CAS PubMed; (b) J. Chan, S. C. Dodani and C. J. Chang, Nat. Chem., 2012, 4, 973 CrossRef CAS PubMed; (c) M. E. Jun, B. Roy and K. H. Ahn, Chem. Commun., 2011, 47, 7583 RSC.
- Y. Hori and K. Kikuchi, Curr. Opin. Chem. Biol., 2013, 17, 644 CrossRef CAS PubMed.
- K. Uno, H. Niikura, M. Morimoto, Y. Ishibashi, H. Miyasaka and M. Irie, J. Am. Chem. Soc., 2011, 133, 13558 CrossRef CAS PubMed.
- (a) M. Irie, T. Fukaminato, K. Matsuda and S. Kobatake, Chem. Rev., 2014, 114, 12174 CAS; (b) B. L. Feringa, Molecular Switches, Wiley-VCH, Weinheim, 2010 Search PubMed; (c) L. Ubaghs, D. Sud and N. R. Branda, Handbook in Thiophene-Based Materials: Applications in Organic Electronics and Photonics, ed. I. D. Perepichka and D. Perepichka, John Wiley & Sons, Chichester, 2009, vol. 2 Search PubMed; (d) H. Tian and S. Yang, Chem. Soc. Rev., 2004, 33, 85 RSC.
- (a) T. Wu, S. Kaur and N. R. Branda, Org. Biomol. Chem., 2015, 13, 2017 Search PubMed; (b) T. Wu, D. Wilson and N. R. Branda, Chem. Mater., 2014, 26, 4313 CrossRef CAS; (c) T. Wu, M. Barker, C.-J. Carling, K. M. Arafeh, J.-C. Boyer and N. R. Branda, Angew. Chem., Int. Ed., 2013, 52, 11106 CrossRef CAS PubMed; (d) T. Wu, J.-C. Boyer, M. Barker, D. Wilson and N. R. Branda, Chem. Mater., 2013, 25, 2495 CrossRef CAS.
- (a) M. Nyk, R. Kumar, T. Y. Ohulchanskyy, E. J. Bergey and P. N. Prasad, Nano Lett., 2008, 8, 3834 CrossRef CAS PubMed; (b) M. Haase and H. Schäfer, Angew. Chem., Int. Ed., 2011, 50, 5808 CrossRef CAS PubMed; (c) S. Gai, C. Li, P. Yang and J. Lin, Chem. Rev., 2014, 114, 2343 CrossRef CAS PubMed; (d) Y. Park, K. T. Lee, Y. D. Suh and T. Hyeon, Chem. Soc. Rev., 2015, 44, 1302 RSC.
- J.-H. Lee and J. Cheon, Anal. Sci. Technol., 2011, 2, A71 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed descriptions of experimental methods, synthetic procedures, characterization of new compounds and additional absorption spectra. See DOI: 10.1039/c5nr03058g |
| ‡ See ESI† for details. |
| § Standard hand-held lamps used for visualizing TLC plates were used to carry out the ring-closing and photolysis reactions at 365 nm (1.4 mW cm−2). Irradiation was carried out in low-light conditions to minimize interference from ambient light. |
| ¶ Visible light irradiation was carried out using the light of a 150 W tungsten source that was passed through a 434 nm cutoff filter to eliminate higher-energy light. Irradiation was carried out in low-light conditions to minimize interference from ambient light. |
| || This value is the total concentration of organic chromophores entrapped within the nano-assemblies obtained using the concentration of the ring-closed isomer (1c) at its photostationary state (as measured by UV-vis spectroscopy) divided by percentage of ring-closed isomers in this state (as measured by 1H NMR spectroscopy). See ESI† for details. |
| ** The quantity of NaN3 buffer in the experiment was carefully controlled to assure paralyzing the nematodes to the level where they were immobile enough to take pictures with good resolution and focus while not killing them. |
| This journal is © The Royal Society of Chemistry 2015 |