Effects of morphology and chemical doping on electrochemical properties of metal hydroxides in pseudocapacitors†
Abstract
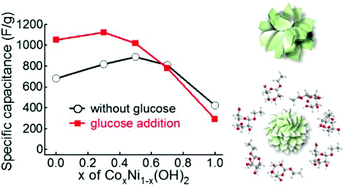
It is well known that both the structural morphology and chemical doping are important factors that affect the properties of metal hydroxide materials in electrochemical energy storage devices. In this work, an effective method to tailor the morphology and chemical doping of metal hydroxides is developed. It is shown that the morphology and the degree of crystallinity of Ni(OH)2 can be changed by adding glucose in the ethanol-mediated solvothermal synthesis. Ni(OH)2 produced in this manner exhibited an increased specific capacitance, which is partially attributed to its increased surface area. Interestingly, the effect of morphology on cobalt doped-Ni(OH)2 is found to be more effective at low cobalt contents than at high cobalt contents in terms of improving the electrochemical performance. This result reveals the existence of competitive effects between chemical doping and morphology change. These findings will provide important insights to design effective materials for energy storage devices.

 Please wait while we load your content...
Please wait while we load your content...