Open Access Article
Open Access ArticleNaF-mediated controlled-synthesis of multicolor NaxScF3+x:Yb/Er upconversion nanocrystals†
Wen-Bo
Pei
a,
Bo
Chen
a,
Lili
Wang
a,
Jiansheng
Wu
b,
Xue
Teng
a,
Raymond
Lau
*a,
Ling
Huang
*c and
Wei
Huang
c
aSchool of Chemical and Biomedical Engineering, Nanyang Technological University, 62 Nanyang Drive, 637459, Singapore. E-mail: WMLau@ntu.edu.sg
bSchool of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Ave, 639672, Singapore
cKey Laboratory of Flexible Electronics (KLOFE) & Institute of Advanced Materials (IAM), Jiangsu National Synergistic Innovation Center for Advanced Materials (SICAM), Nanjing Tech University (NanjingTech), 30 South Puzhu Road, Nanjing, 211816, P.R. China. E-mail: iamlhuang@njtech.edu.cn
First published on 27th January 2015
Abstract
Synthesis of lanthanide-doped upconversion nanocrystals (LDUNs) with controlled morphology and luminescence has long been desired in order to fulfill various application requirements. In this work, we have investigated the effect of the NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio, in the range of 1 to 20, on the morphology, crystal structure, and upconversion properties of NaxScF3+x:Yb/Er nanocrystals that are reported to possess different upconversion properties from those of NaYF4:Yb/Er nanocrystals. The experimental results prove that the NaF
Ln3+ molar ratio, in the range of 1 to 20, on the morphology, crystal structure, and upconversion properties of NaxScF3+x:Yb/Er nanocrystals that are reported to possess different upconversion properties from those of NaYF4:Yb/Er nanocrystals. The experimental results prove that the NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio influences significantly the growth process of the nanocrystals, i.e. a low NaF
Ln3+ molar ratio influences significantly the growth process of the nanocrystals, i.e. a low NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio results in hexagonal NaScF4 nanocrystals, while a high NaF
Ln3+ molar ratio results in hexagonal NaScF4 nanocrystals, while a high NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio favors monoclinic Na3ScF6 nanocrystals. When the NaF
Ln3+ molar ratio favors monoclinic Na3ScF6 nanocrystals. When the NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio is as high as 6 or above, phase separation is found and hexagonal NaYbF4 nanocrystals showed up for the first time. Simply by adjusting the NaF
Ln3+ molar ratio is as high as 6 or above, phase separation is found and hexagonal NaYbF4 nanocrystals showed up for the first time. Simply by adjusting the NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio, we have successfully achieved the simultaneous control of the shape, size, as well as the crystallographic phase of the NaxScF3+x:Yb/Er nanocrystals, which give different red to green (R/G) ratios (integral area), leading to a multicolor upconversion luminescence from orange-red to green. This study provides a vivid example to track and interpret the formation mechanisms and growth processes of NaxScF3+x:Yb/Er nanocrystals, which shall be instructive for guiding the controlled synthesis of other LDUNs and extending their according applications in optical communication, color display, anti-counterfeiting, bioimaging, and so on.
Ln3+ molar ratio, we have successfully achieved the simultaneous control of the shape, size, as well as the crystallographic phase of the NaxScF3+x:Yb/Er nanocrystals, which give different red to green (R/G) ratios (integral area), leading to a multicolor upconversion luminescence from orange-red to green. This study provides a vivid example to track and interpret the formation mechanisms and growth processes of NaxScF3+x:Yb/Er nanocrystals, which shall be instructive for guiding the controlled synthesis of other LDUNs and extending their according applications in optical communication, color display, anti-counterfeiting, bioimaging, and so on.
1 Introduction
Photon upconversion (UC) has attracted increasing research interest for decades since its first recognition and formulation by Auzel and Ovsyankin in the mid-1960s,1–5 whereas lanthanide ion (Ln3+) doped luminescent materials have shown an excellent near infrared (NIR) to visible UC efficiency, with unparalleled advantages, including but not limited to narrow emission bands, long luminescence lifetimes (micro- to millisecond range), low cytotoxicity, and high resistance to photobleaching, photoblinking and photochemical degradation.6,7 These unique characteristics have offered them wide applications in areas ranging from high-resolution displays, integrated optical systems, substitutes for organic dyes, solid-state lasers, to biological labels, optical communication, and so on.8–18Among all the lanthanide-based host materials observed to date including oxides, phosphates, and vanadates,19–23 fluorides are proved to be the most efficient hosts for visible UC luminescence, due to their intrinsically low phonon energies, which lead to a decrease in the nonradiative relaxation rate that affects the UC efficiency.24–33 NaMF4:Yb/Ln (M = Y, La, Gd or Lu, Ln = Er or Tm) UC nanocrystals showing various crystal structures, morphology as well as UC luminescence have been systematically investigated in the last 10 years.9,34–38 The effects of the experimental factors such as the reaction time and temperature, the chemical composition and the polarity of the solvents, the F−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio, as well as the synthesis procedure on the structure and dimensions of the NaMF4:Yb/Ln nanocrystals have been studied extensively.39–41
Ln3+ molar ratio, as well as the synthesis procedure on the structure and dimensions of the NaMF4:Yb/Ln nanocrystals have been studied extensively.39–41
However, except few recent reports on the synthesis and UC luminescence of NaxScF3+x nanocrystals, there is a lack of a systematic study on the controlled synthesis of NaxScF3+x nanocrystals under varying experimental conditions.42,43 What is more, since Sc3+ possesses the smallest rare earth ionic radius and distinct atomic electronic configuration, Sc3+ ion-based UC nanocrystals usually give a strong red UC luminescence at 660 nm,44 which is different from those of NaMF4:Yb/Er (M = Y, La, Gd or Lu) nanocrystals. Thus, it is even worth carrying out a systematic exploration of the effect of varying experimental conditions on the obtained NaxScF3+x nanocrystals and further on their UC luminescence.
Suitable applications normally require the UC nanocrystals to have precise parameters, such as size, shape, crystallographic phase, chemical composition, and the desired luminescence properties. In addition, the nanocrystals should be uniformly shaped while maintaining a high-monodispersion and well-defined crystalline structure. Hence, controllable synthesis of the nanocrystals with the desired parameters and UC luminescence has long been remained a challenging topic,45–47 and a comprehensive understanding of the process of nanocrystal growth and the cause of phase transition will provide straightforward clues to solve such challenges.
In this paper, a controlled synthesis of NaxScF3+x:Yb/Er nanocrystals at different shapes, sizes, and crystallographic phases is achieved simply by adjustment of the NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio. In addition, phase separation and hexagonal NaYbF4 nanocrystals are observed at a higher NaF
Ln3+ molar ratio. In addition, phase separation and hexagonal NaYbF4 nanocrystals are observed at a higher NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio for the first time. The according UC luminescence of each product and the possible energy transfer mechanism are also discussed.
Ln3+ molar ratio for the first time. The according UC luminescence of each product and the possible energy transfer mechanism are also discussed.
2 Experimental section
2.1 Chemicals and materials
All the starting chemicals and reagents used in the experiments, including ScCl3·6H2O (99.99%), YbCl3·6H2O (99.9%), ErCl3·6H2O (99.9%), NaF(99%), 1-octadecene (90%), oleic acid (90%), alcohol (95%), and cyclohexane (99.9%) were purchased from Sigma-Aldrich and used as received.2.2 Nanocrystal synthesis
1 mmol RECl3·6H2O (0.78 mmol ScCl3·6H2O, 0.2 mmol YbCl3·6H2O, 0.02 mmol ErCl3·6H2O) was added to a 50 mL flask containing 12.5 mL oleic acid and 12.5 mL 1-octadecene. The solution was heated to 160 °C under argon protection. Subsequently, an appropriate amount of NaF (1, 1.5, 2, 2.5, 4, 5, 6, 8, 10, 14 and 20 mmol) according to the desired product phase and chemical composition, was added directly to the solution. After 30 minutes of stirring, the solution was heated to 300 °C directly under an argon environment for 1.5 h with vigorous magnetic stirring. Ethanol was added to the solution after cooling down to room temperature, and the resulting nanocrystals were collected by centrifugation, washed with the mixture of water and ethanol several times, and finally re-dispersed in cyclohexane.2.3 Characterization
The morphology and structure of the nanocrystals were characterized by using a low resolution (JEOL JEM-1400) transmission electron microscope (TEM) operated at an accelerating voltage of 100 kV and high resolution TEM (HRTEM) (JEOL JEM-3010) operated at an accelerating voltage of 300 kV. Energy Dispersive X-ray Spectrometry (EDS) measurement was collected using the JED-2300 Analysis Station operated at 20 kV. The crystallographic information of the samples was obtained by the X-ray diffraction (XRD) measurements, using a Bruker D2 Phaser XRD with Cu Kα radiation (λ = 1.5406 Å) from 10° to 70° at a step of 0.1° s−1. The UC luminescence spectra were recorded on a Horiba Jobin Yvon FluoroMax-4 system and an external MDL/MDL-H-980 nm CW laser system was used as the excitation source. The nanocrystals were dispersed in cyclohexane (1 wt%) in a standard quartz cuvette at room temperature to measure the UC luminescence spectra.3 Results and discussion
TEM images of the NaxScF3+x:Yb/Er nanocrystals synthesized at NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratios of 1 (a), 1.5 (b), 2 (c), 2.5 (d), 4 (e) and 5 (f) are shown in Fig. 1. It can be seen that the shape of the nanocrystals synthesized at different NaF
Ln3+ molar ratios of 1 (a), 1.5 (b), 2 (c), 2.5 (d), 4 (e) and 5 (f) are shown in Fig. 1. It can be seen that the shape of the nanocrystals synthesized at different NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ ratios changes from nanospheres at the low NaF
Ln3+ ratios changes from nanospheres at the low NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ ratio, i.e., 1, to the mixture of both nanospheres and nanocubes at the NaF
Ln3+ ratio, i.e., 1, to the mixture of both nanospheres and nanocubes at the NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ ratio of 1.5, then big nanorods, nanospheres, nanocubes and eventually small nanopolyhedra at higher NaF
Ln3+ ratio of 1.5, then big nanorods, nanospheres, nanocubes and eventually small nanopolyhedra at higher NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratios of 2, 2.5, 4 and 5 respectively. Moreover, the size of the obtained nanocrystals increases gradually from 18.2 nm to 57.6 nm as the NaF
Ln3+ molar ratios of 2, 2.5, 4 and 5 respectively. Moreover, the size of the obtained nanocrystals increases gradually from 18.2 nm to 57.6 nm as the NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio increases from 1 (a) to 4 (e), and then sharply decreases at the NaF
Ln3+ molar ratio increases from 1 (a) to 4 (e), and then sharply decreases at the NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ ratio of 5 (f). The results clearly demonstrate that the NaF
Ln3+ ratio of 5 (f). The results clearly demonstrate that the NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio, which is responsible for the shape and size evolution, has a great impact on the dynamic process governing nucleation and growth of the NaxScF3+x nanocrystals.
Ln3+ molar ratio, which is responsible for the shape and size evolution, has a great impact on the dynamic process governing nucleation and growth of the NaxScF3+x nanocrystals.
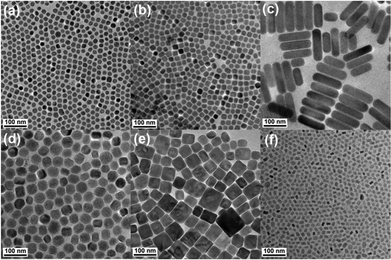 | ||
Fig. 1 TEM images of NaxScF3+x:Yb/Er nanocrystals synthesized at NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratios of 1 (a), 1.5 (b), 2 (c), 2.5 (d), 4 (e) and 5 (f), respectively. Ln3+ molar ratios of 1 (a), 1.5 (b), 2 (c), 2.5 (d), 4 (e) and 5 (f), respectively. | ||
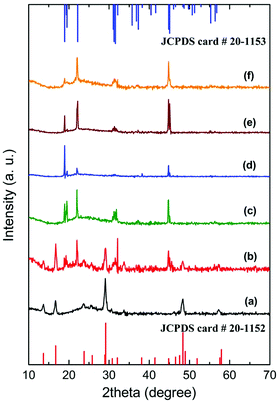
XRD patterns were collected and shown in Fig. 2 to confirm the crystallographic phases of the nanocrystals synthesized under each individual experimental conditions. It can be found that pure hexagonal phase NaScF4 (JCPDS 20-1152) nanocrystals can only be observed at the NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio of 1 (a), while pure monoclinic phase Na3ScF6 (JCPDS 20-1153) nanocrystals can be collected at a wide NaF
Ln3+ molar ratio of 1 (a), while pure monoclinic phase Na3ScF6 (JCPDS 20-1153) nanocrystals can be collected at a wide NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio range condition from 2 to 5 (c, d, e, f). A moderate NaF
Ln3+ molar ratio range condition from 2 to 5 (c, d, e, f). A moderate NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio of 1.5 results in a mixture of both hexagonal and monoclinic phases (b). HRTEM images of the resulting NaScF4:Yb/Er and Na3ScF6:Yb/Er nanocrystals are shown in Fig. S1.† Lattice fringes of NaScF4:Yb/Er and Na3ScF6:Yb/Er nanocrystals were observed to have a d-spacing of 0.307 nm and 0.402 nm respectively, corresponding to the distance of the (221) planes of NaScF4 and (110) planes of the Na3ScF6 phase. Lattice parameters for both hexagonal and monoclinic NaxScF3+x:Yb/Er phases were calculated based on the XRD patterns. The results show the cell parameters of a = 13.0548 Å, c = 9.2099 Å and V = 1359.33 Å3 for hexagonal as well as a = 5.6053 Å, b = 5.8520 Å, c = 8.0867 Å, and V = 265.25 Å3 for the monoclinic phase. Both cell volumes are slightly higher than those of the standard data (V = 1350.49 Å3 and 264.17 Å3 respectively), which indicates the formation of Yb/Er doped NaxScF3+x nanocrystals because the Yb3+ and Er3+ ions of the larger ionic radii partially substitute the Sc3+ ions of smaller ionic radii. Composition analysis of the as-prepared NaxScF3+x:Yb/Er nanocrystals by using EDS reveals that the nanocrystals are mainly composed of Na, Sc, Yb, F and a small amount of Er element. It confirms the successful doping of Yb3+ and Er3+ ions in the host lattice in a direct manner (Fig. S2†).
Ln3+ molar ratio of 1.5 results in a mixture of both hexagonal and monoclinic phases (b). HRTEM images of the resulting NaScF4:Yb/Er and Na3ScF6:Yb/Er nanocrystals are shown in Fig. S1.† Lattice fringes of NaScF4:Yb/Er and Na3ScF6:Yb/Er nanocrystals were observed to have a d-spacing of 0.307 nm and 0.402 nm respectively, corresponding to the distance of the (221) planes of NaScF4 and (110) planes of the Na3ScF6 phase. Lattice parameters for both hexagonal and monoclinic NaxScF3+x:Yb/Er phases were calculated based on the XRD patterns. The results show the cell parameters of a = 13.0548 Å, c = 9.2099 Å and V = 1359.33 Å3 for hexagonal as well as a = 5.6053 Å, b = 5.8520 Å, c = 8.0867 Å, and V = 265.25 Å3 for the monoclinic phase. Both cell volumes are slightly higher than those of the standard data (V = 1350.49 Å3 and 264.17 Å3 respectively), which indicates the formation of Yb/Er doped NaxScF3+x nanocrystals because the Yb3+ and Er3+ ions of the larger ionic radii partially substitute the Sc3+ ions of smaller ionic radii. Composition analysis of the as-prepared NaxScF3+x:Yb/Er nanocrystals by using EDS reveals that the nanocrystals are mainly composed of Na, Sc, Yb, F and a small amount of Er element. It confirms the successful doping of Yb3+ and Er3+ ions in the host lattice in a direct manner (Fig. S2†).
The crystallographic phases agree well with the morphology evolution observed in Fig. 1. As is known that nanocrystals with a hexagonal phase usually give hexagonal plates or hexagonal prisms in an external format, while a monoclinic phase shows rod or block shaped mostly. Sphere-like to some extent, is a characteristic feature of small polyhedra under the TEM measurement. Obviously, the crystallographic phase evolution from hexagonal phases to mixed phases, and then the monoclinic phase gives a reasonable explanation for the shape development of the NaxScF3+x:Yb/Er nanocrystals. It is also noticed that the intensity of the relative diffraction peaks for the monoclinic phase in the XRD patterns switches with the morphologies evolution, suggesting the existence of preferential orientation as well as the versatile morphologies.
The above results indicate clearly that, at a low NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio of 1, the hexagonal phase NaScF4 is formed, while at a high NaF
Ln3+ molar ratio of 1, the hexagonal phase NaScF4 is formed, while at a high NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio (2–5), the monoclinic phase Na3ScF6 is favored. It is reported that the growth of the as-prepared NaxScF3+x:Yb/Er nanocrystal started with the reaction between F− ions and rare earth oleate complexes.48 A high F− content can significantly accelerate the particle nucleation rate. A much faster nucleation speed results in a thermodynamically determined reaction process.45 The corresponding thermodynamically stable phase monoclinic Na3ScF6 nanocrystals are formed. The size and morphology of the resulting nanocrystals are dependent on the dynamic process. The capping effect of F− on the crystal surface would alter the average number of dangling bonds, and further change the chemical potential of the crystal, as well as the crystal plane.45 Different NaF contents involved in each reaction conditions will cause the relative growth rate on different directions changing accordingly, leading to different crystal morphologies and sizes. In contrast, at a low NaF
Ln3+ molar ratio (2–5), the monoclinic phase Na3ScF6 is favored. It is reported that the growth of the as-prepared NaxScF3+x:Yb/Er nanocrystal started with the reaction between F− ions and rare earth oleate complexes.48 A high F− content can significantly accelerate the particle nucleation rate. A much faster nucleation speed results in a thermodynamically determined reaction process.45 The corresponding thermodynamically stable phase monoclinic Na3ScF6 nanocrystals are formed. The size and morphology of the resulting nanocrystals are dependent on the dynamic process. The capping effect of F− on the crystal surface would alter the average number of dangling bonds, and further change the chemical potential of the crystal, as well as the crystal plane.45 Different NaF contents involved in each reaction conditions will cause the relative growth rate on different directions changing accordingly, leading to different crystal morphologies and sizes. In contrast, at a low NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio, which means a low F− content, nucleation and nanocrystal growing becomes quite slow and metastable hexagonal phase NaScF4 nanocrystals are formed.
Ln3+ molar ratio, which means a low F− content, nucleation and nanocrystal growing becomes quite slow and metastable hexagonal phase NaScF4 nanocrystals are formed.
NaxScF3+x:Yb/Er nanocrystals prepared at much higher NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratios, i.e., 6, 8, 10, 14 and 20 were also investigated and the corresponding TEM images are shown in Fig. 3 and S3.† Different from the previous ones which show quite uniform morphologies, a mixture of both small nanoparticles and big hexagonal plates was found. As the NaF
Ln3+ molar ratios, i.e., 6, 8, 10, 14 and 20 were also investigated and the corresponding TEM images are shown in Fig. 3 and S3.† Different from the previous ones which show quite uniform morphologies, a mixture of both small nanoparticles and big hexagonal plates was found. As the NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio increases from 6 to 8, and further to 10, besides a small quantity of big hexagonal plates with a mean size of 150 nm in diameter and 80 nm in thickness is observed within the samples (Fig. 3d), and the rest of the majority of small nanocrystals decreases from 18.0 nm (Fig. 3a) to 10.5 nm (Fig. 3b), and finally to 8.2 nm (Fig. 3c). Along with the NaF
Ln3+ molar ratio increases from 6 to 8, and further to 10, besides a small quantity of big hexagonal plates with a mean size of 150 nm in diameter and 80 nm in thickness is observed within the samples (Fig. 3d), and the rest of the majority of small nanocrystals decreases from 18.0 nm (Fig. 3a) to 10.5 nm (Fig. 3b), and finally to 8.2 nm (Fig. 3c). Along with the NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio further increasing, i.e., 14 and 20, the tiny nanocrystals reach a minimum diameter at about 8.0 nm (Fig. S3†) and remain constant. Nanocrystals with such obviously different morphologies observed in one sample are never reported in NaxScF3+x:Yb/Er nanocrystals, which may indicate the mixture of dramatically different crystal phases. XRD patterns shown in Fig. S4† prove the mixture of both monoclinic phase Na3ScF6 and hexagonal phase NaYbF4 nanocrystals. Obviously, the majority of small nanoparticles belong to the Na3ScF6 nanocrystals, while the big hexagonal plates are the NaYbF4 nanocrystals. The broadening peak width for Na3ScF6 nanocrystals as well as the narrow peak width for the NaYbF4 crystal phase observed from the XRD patterns gives an indirect evidence illustrating the conclusion. Such a particular phase separation phenomenon has never been reported before. Comparison experiments for NaMF4:Yb/Er (M = Y and Gd) nanocrystals synthesized at a NaF
Ln3+ molar ratio further increasing, i.e., 14 and 20, the tiny nanocrystals reach a minimum diameter at about 8.0 nm (Fig. S3†) and remain constant. Nanocrystals with such obviously different morphologies observed in one sample are never reported in NaxScF3+x:Yb/Er nanocrystals, which may indicate the mixture of dramatically different crystal phases. XRD patterns shown in Fig. S4† prove the mixture of both monoclinic phase Na3ScF6 and hexagonal phase NaYbF4 nanocrystals. Obviously, the majority of small nanoparticles belong to the Na3ScF6 nanocrystals, while the big hexagonal plates are the NaYbF4 nanocrystals. The broadening peak width for Na3ScF6 nanocrystals as well as the narrow peak width for the NaYbF4 crystal phase observed from the XRD patterns gives an indirect evidence illustrating the conclusion. Such a particular phase separation phenomenon has never been reported before. Comparison experiments for NaMF4:Yb/Er (M = Y and Gd) nanocrystals synthesized at a NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio of 10 were done under the same process. XRD patterns shown in Fig. S5† demonstrate clearly that no phase separation phenomenon can be observed in the Y3+/Gd3+ ion-based UC systems. Thus, phase separation is a particular phenomenon in the Sc3+ ion-based UC system. Considering that there is a big difference of rare earth ionic radius of Sc and doping ions, phase separation may occur under the condition when there is a quite fast nucleation rate at a much higher NaF content. Furthermore, crystallographic phase difference between NaxScF3+x and NaxYbFy systems should be another important reason. The monoclinic phase Na3ScF6 is formed at a high NaF
Ln3+ molar ratio of 10 were done under the same process. XRD patterns shown in Fig. S5† demonstrate clearly that no phase separation phenomenon can be observed in the Y3+/Gd3+ ion-based UC systems. Thus, phase separation is a particular phenomenon in the Sc3+ ion-based UC system. Considering that there is a big difference of rare earth ionic radius of Sc and doping ions, phase separation may occur under the condition when there is a quite fast nucleation rate at a much higher NaF content. Furthermore, crystallographic phase difference between NaxScF3+x and NaxYbFy systems should be another important reason. The monoclinic phase Na3ScF6 is formed at a high NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio. However, there is no monoclinic phase found for the NaxYbFy system. Phase separation under the high NaF:Ln3+ molar ratio condition is realized.
Ln3+ molar ratio. However, there is no monoclinic phase found for the NaxYbFy system. Phase separation under the high NaF:Ln3+ molar ratio condition is realized.
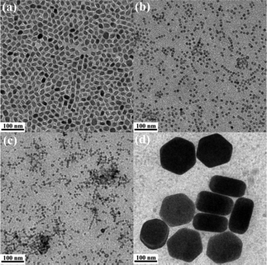 | ||
Fig. 3 TEM images of NaxScF3+x:Yb/Er nanocrystals synthesized at NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratios of 6 (a), 8 (b), 10 (c) and the corresponding NaYbF4 hexagonal plates (d), respectively. Ln3+ molar ratios of 6 (a), 8 (b), 10 (c) and the corresponding NaYbF4 hexagonal plates (d), respectively. | ||
The findings clearly show that the NaF content can influence the growth process significantly and allow simultaneous control of the shape, size, as well as the crystallographic phase of the NaxScF3+x:Yb/Er nanocrystals. In other words, a simple tuning of the NaF content can be used to synthesize NaxScF3+x:Yb/Er nanocrystals with a controllable morphology and a crystallographic phase. Furthermore, phase separation is found and investigated for the first time in the NaxScF3+x:Yb/Er nanocrystals system.
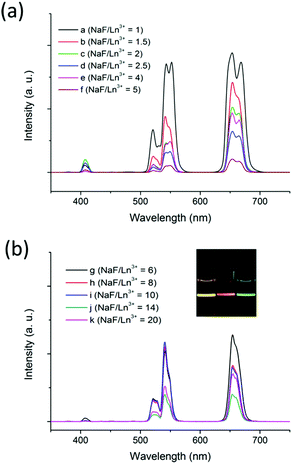
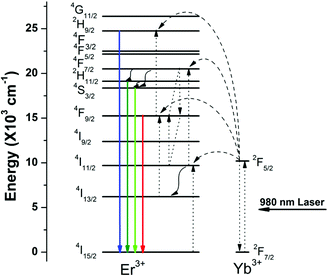
To investigate the effect of the nanocrystal morphology and phase on the UC emission of the NaxScF3+x:Yb/Er nanocrystals, luminescence spectra were collected under 980 nm laser excitation. In the UC spectra shown in Fig. 4, three characteristic peaks located at 408, 541, and 654 nm can be found in all the samples. The emission band centered at 408 nm can be ascribed to the 2H9/2 → 4I15/2 transition of Er3+ ions. Green UC emissions at 510–560 nm and red UC emission at 640–670 nm can be ascribed to 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transitions and Er3+ 4F9/2 → 4I15/2 transition of Er3+, respectively.49,50Fig. 5 shows the details of the UC energy transfer mechanism of NaxScF3+x:Yb/Er nanocrystals.
It is noticed that all the NaxScF3+x:Yb/Er nanocrystals show higher R/G ratios than those of NaYF4:Yb/Er nanocrystals. It is found that the latter ones generally give strong green emission with a small R/G ratio (Table 1 and S6†), while Sc3+ ion-based nanocrystals show distinctive UC emission with enhanced red UC emission due to the small radius of the Sc3+ ion.44 The small radius of the Sc3+ ion results in a shorter Sc3+–Sc3+ distance compared to that of Y3+–Y3+ with similar fluoride bridged moieties inside the host crystal. A short Sc3+–Sc3+ distance leads to a short doping ions distance of Yb3+–Er3+ cation-pairs. On the other hand, a Ln3+ (Yb3+ and Er3+ ions) cluster may exist because of the strong structural inhomogeneity due to the large cationic radius difference between Sc3+ and Ln3+. The short distance between Ln3+ ions within NaxScF3+x host nanocrystals could enhance the cross relaxation (4F7/2 + 4I11/2→4F9/2 + 4F9/2). It diminishes the population in 2H11/2 and 4S3/2 levels and enhances the population in the 4F9/2 energy level of Er3+. Consequently, the NaxScF3+x:Yb/Er nanocrystals show enhanced red emission centered at 660 nm and the R/G ratio becomes higher than that of NaYF4 host nanophosphors.
NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ Ln3+ |
Shape | Size (NaxScF3+x) nm−1 | Crystallographic phase | R/G ratio |
|---|---|---|---|---|
| 1 | Nanosphere | 18.2 ± 1.0 | Hexagonal phase (H) | 1.2852 |
| 1.5 | Nanosphere & Nanocube | 23.0 ± 0.8 | Mixture of H and M | 1.8333 |
| 2 | Nanorod | (138.5 ± 2.5) × (39.4 ± 1.2) | Monoclinic phase (M) | 2.4379 |
| 2.5 | Nanosphere | 43.5 ± 1.6 | M | 2.2500 |
| 4 | Nanocube | 57.6 ± 5.7 | M | 2.2055 |
| 5 | Nanopolyhedron | 17.8 ± 1.1 | M | 2.1657 |
| 6 | Nanopolyhedron & Hexagonal plate | 18.0 ± 0.7 | Mixture of M and NaYbF4 | 1.3681 |
| 8 | Tiny particle & hexagonal plate | 10.5 ± 0.3 | Mixture of M and NaYbF4 | 0.8184 |
| 10 | Tiny particle & hexagonal plate | 8.2 ± 0.4 | Mixture of M and NaYbF4 | 0.7337 |
| 14 | Tiny particle & hexagonal plate | 8.0 ± 0.3 | Mixture of M and NaYbF4 | 1.0743 |
| 20 | Tiny particle & hexagonal plate | 8.0 ± 0.3 | Mixture of M and NaYbF4 | 1.4063 |
Moreover, it is worth noting that multicolor UC luminescence can be successfully realized in the NaxScF3+x:Yb/Er nanocrystals (inset of Fig. 4). Hexagonal phase NaScF4:Yb/Er nanocrystals give bright yellow UC luminescence with the R/G ratio of 1.2852 and the overall UC intensity is obviously stronger than that of monoclinic Na3ScF6:Yb/Er ones. The monoclinic phase Na3ScF6:Yb/Er nanocrystals show orange-red color UC luminescence with a R/G ratio >2.16, while the mixed phases of both Na3ScF6 and NaYbF4 show green color UC luminescence with a small R/G ratio, i.e. 0.7337. The results agree well with what have been reported before.43,44,51 As there exists multiple independent sites for both Yb3+ and Er3+ in the hexagonal phase NaScF4, which could increase the number of possible Yb3+ to Er3+ energy transfer processes, especially for the 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transition processes. Thus a much stronger bright yellow color UC luminescence is achieved. For the monoclinic Na3ScF6:Yb/Er nanostructures, the R/G ratios are similar for the four samples and all show similar orange-red color UC luminescence. However, the overall UC intensity is different. The nanorods and nanocubes give the strongest UC luminescence, then the nanospheres and finally the nanopolyhedra. The results are reasonable as nanocrystals with good crystallinity and larger size, implying fewer surface defects and more luminescent centers deeply inside, could enable the luminescence processes more effectively. As for the green color UC luminescence found within the mixed phases of both Na3ScF6 and NaYbF4, it is believed that the green color emission mainly arose from the Na3ScF6 nanocrystals with a low doping level of Yb and Er ions. A comparison experiment for NaYbF4:Er nanocrystals was performed initially and it showed yellow color UC luminescence (Fig. S7†). However, it is reported that low doping levels of Yb and Er ions, i.e., 1 mol% Er3+/2 mol% Yb3+ codoped Na3ScF6 microcrystals show strong green UC luminescence.52 Moreover, separate phases of the ultra-small Na3ScF6:Yb/Er and large NaYbF4:Er nanocrystals for the sample at a NaF/Ln3+ molar ratio of 10 were collected for UC luminescence comparison and the results are shown in Fig. S8.† It can be seen that the ultra-small Na3ScF6:Yb/Er nanocrystals showed stronger emission with an R/G ratio of 0.7064 and gave green color emission; while the large NaYbF4:Er nanocrystals showed weaker emission with an R/G ratio of 0.9564 and gave yellow color emission. As the amount of NaYbF4 is quite low, mixed phases of a large amount of Na3ScF6:Yb/Er and a small amount of NaYbF4:Er nanocrystals showed an overall green color emission with an R/G ratio of 0.7337 and the green color emission mainly arose from the UC luminescence contribution of the Na3ScF6:Yb/Er nanocrystals. As the NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio was further increased beyond 10, much more NaYbF4:Er were formed gradually and the R/G ratio was increased. When the NaF
Ln3+ molar ratio was further increased beyond 10, much more NaYbF4:Er were formed gradually and the R/G ratio was increased. When the NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio reached 20, nearly no Yb and Er ions were embedded into the Na3ScF6 host lattice; yellow color UC luminescence originated from the NaYbF4:Er nanocrystals.
Ln3+ molar ratio reached 20, nearly no Yb and Er ions were embedded into the Na3ScF6 host lattice; yellow color UC luminescence originated from the NaYbF4:Er nanocrystals.
4 Conclusions
NaxScF3+x:Yb/Er nanocrystals with controllable shape (nanospheres, nanorods, nanocubes and nanopolyhedra), size (from hundreds of nanometers to sub-ten nm), and crystallographic phase (monoclinic, hexagonal and their mixture) are synthesized simply by the adjustment of the NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio in the range of 1 to 20. Phase separation is observed for the first time at a high NaF
Ln3+ molar ratio in the range of 1 to 20. Phase separation is observed for the first time at a high NaF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ln3+ molar ratio range (>6). The NaxScF3+x:Yb/Er nanocrystals show a higher R/G ratio than those of NaYF4:Yb/Er. More importantly, multicolor emissions from orange-red, yellow to green can be achieved, which demonstrates that this series of host materials, NaxScF3+x, has promising applications in optical communication, high resolution three-dimensional bioimaging, color displays, solid-state lasers, photocatalysis, and photodynamic therapy.
Ln3+ molar ratio range (>6). The NaxScF3+x:Yb/Er nanocrystals show a higher R/G ratio than those of NaYF4:Yb/Er. More importantly, multicolor emissions from orange-red, yellow to green can be achieved, which demonstrates that this series of host materials, NaxScF3+x, has promising applications in optical communication, high resolution three-dimensional bioimaging, color displays, solid-state lasers, photocatalysis, and photodynamic therapy.
Acknowledgements
R. L. thanks the financial support from NEA ETRP Grant (ref no.: 1102 108). L. H. is grateful for the financial support from the National Natural Science Foundation of China (grant no. 21379105). It is also supported by the China Postdoctoral Science Foundation (grant no. 2013M541655) and Jiangsu Planned Projects for Postdoctoral Research Funds (grant no. 1301039B).Notes and references
- F. Auzel, Chem. Rev., 2003, 104, 139–174 CrossRef PubMed.
- S. Gai, C. Li, P. Yang and J. Lin, Chem. Rev., 2014, 114, 2343–2389 CrossRef CAS PubMed.
- F. Auzel, J. Lumin., 1990, 45, 341–345 CrossRef CAS.
- M. Haase and H. Schäfer, Angew. Chem., Int. Ed., 2011, 50, 5808–5829 CrossRef CAS PubMed.
- P. P. Feofilov and V. V. Ovsyankin, Appl. Opt., 1967, 6, 1828–1833 CrossRef CAS PubMed.
- G. Wang, Q. Peng and Y. Li, Acc. Chem. Res., 2011, 44, 322–332 CrossRef CAS PubMed.
- L. Cheng, C. Wang and Z. Liu, Nanoscale, 2013, 5, 23–37 RSC.
- J. Zhou, Z. Liu and F. Li, Chem. Soc. Rev., 2012, 41, 1323–1349 RSC.
- F. Chen, W. Bu, S. Zhang, J. Liu, W. Fan, L. Zhou, W. Peng and J. Shi, Adv. Funct. Mater., 2013, 23, 298–307 CrossRef CAS.
- L.-L. Li, P. Wu, K. Hwang and Y. Lu, J. Am. Chem. Soc., 2013, 135, 2411–2414 CrossRef CAS PubMed.
- S. Zeng, J. Xiao, Q. Yang and J. Hao, J. Mater. Chem., 2012, 22, 9870–9874 RSC.
- J. Wu, Q. Tian, H. Hu, Q. Xia, Y. Zou, F. Li, T. Yi and C. Huang, Chem. Commun., 2009, 4100–4102 RSC.
- J. Zhou, X. Zhu, M. Chen, Y. Sun and F. Li, Biomaterials, 2012, 33, 6201–6210 CrossRef CAS PubMed.
- X.-F. Qiao, J.-C. Zhou, J.-W. Xiao, Y.-F. Wang, L.-D. Sun and C.-H. Yan, Nanoscale, 2012, 4, 4611–4623 RSC.
- F. Wang, R. Deng, J. Wang, Q. Wang, Y. Han, H. Zhu, X. Chen and X. Liu, Nat. Mater., 2011, 10, 968–973 CrossRef CAS PubMed.
- Y. Yang, F. Liu, X. Liu and B. Xing, Nanoscale, 2013, 5, 231–238 RSC.
- L. Wang, J. Liu, Y. Dai, Q. Yang, Y. Zhang, P. Yang, Z. Cheng, H. Lian, C. Li, Z. Hou, P. a. Ma and J. Lin, Langmuir, 2014, 30, 13042–13051 CrossRef CAS PubMed.
- D. Yang, Y. Dai, J. Liu, Y. Zhou, Y. Chen, C. Li, P. a. Ma and J. Lin, Biomaterials, 2014, 35, 2011–2023 CrossRef CAS PubMed.
- F. Wang and X. Liu, Chem. Soc. Rev., 2009, 38, 976–989 RSC.
- Z.-G. Yan and C.-H. Yan, J. Mater. Chem., 2008, 18, 5046–5059 RSC.
- N. Bogdan, F. Vetrone, G. A. Ozin and J. A. Capobianco, Nano Lett., 2011, 11, 835–840 CrossRef CAS PubMed.
- F. Wang, X. Xue and X. Liu, Angew. Chem., Int. Ed., 2008, 47, 906–909 CrossRef CAS PubMed.
- K. Li, M. Shang, D. Geng, H. Lian, Y. Zhang, J. Fan and J. Lin, Inorg. Chem., 2014, 53, 6743–6751 CrossRef CAS PubMed.
- C. Li and J. Lin, J. Mater. Chem., 2010, 20, 6831–6847 RSC.
- S. Heer, K. Kömpe, H. U. Güdel and M. Haase, Adv. Mater., 2004, 16, 2102–2105 CrossRef CAS.
- S. Sivakumar, F. C. J. M. van Veggel and M. Raudsepp, J. Am. Chem. Soc., 2005, 127, 12464–12465 CrossRef CAS PubMed.
- L. Wang, M. Lan, Z. Liu, G. Qin, C. Wu, X. Wang, W. Qin, W. Huang and L. Huang, J. Mater. Chem. C, 2013, 1, 2485–2490 RSC.
- K. Deng, T. Gong, L. Hu, X. Wei, Y. Chen and M. Yin, Opt. Express, 2011, 19, 1749–1754 CrossRef CAS PubMed.
- F. Liu, Q. Zhao, H. You and Z. Wang, Nanoscale, 2013, 5, 1047–1053 RSC.
- S. Schietinger, T. Aichele, H.-Q. Wang, T. Nann and O. Benson, Nano Lett., 2009, 10, 134–138 CrossRef PubMed.
- J.-C. Boyer, J. Gagnon, L. A. Cuccia and J. A. Capobianco, Chem. Mater., 2007, 19, 3358–3360 CrossRef CAS.
- Q. Xiao, Y. Ji, Z. Xiao, Y. Zhang, H. Lin and Q. Wang, Chem. Commun., 2013, 49, 1527–1529 RSC.
- C. Li, Z. Hou, C. Zhang, P. Yang, G. Li, Z. Xu, Y. Fan and J. Lin, Chem. Mater., 2009, 21, 4598–4607 CrossRef CAS.
- H. H. Gorris and O. S. Wolfbeis, Angew. Chem., Int. Ed., 2013, 52, 3584–3600 CrossRef CAS PubMed.
- B. Zhou, Y. Idobata, A. Kobayashi, H. Cui, R. Kato, R. Takagi, K. Miyagawa, K. Kanoda and H. Kobayashi, J. Am. Chem. Soc., 2012, 134, 12724–12731 CrossRef CAS PubMed.
- G. Tian, Z. Gu, L. Zhou, W. Yin, X. Liu, L. Yan, S. Jin, W. Ren, G. Xing, S. Li and Y. Zhao, Adv. Mater., 2012, 24, 1226–1231 CrossRef CAS PubMed.
- S. Gai, C. Li, P. Yang and J. Lin, Chem. Rev., 2013, 114, 2343–2389 CrossRef PubMed.
- H. Na, J. Jeong, H. Chang, H. Kim, K. Woo, K. Lim, K. A. Mkhoyan and H. Jang, Nanoscale, 2014, 6, 7461–7468 RSC.
- Y. Liu, D. Tu, H. Zhu and X. Chen, Chem. Soc. Rev., 2013, 42, 6924–6958 RSC.
- F. Wang and X. Liu, Acc. Chem. Res., 2014, 47, 1378–1385 CrossRef CAS PubMed.
- H. Na, K. Woo, K. Lim and H. S. Jang, Nanoscale, 2013, 5, 4242–4251 RSC.
- Y. Ai, D. Tu, W. Zheng, Y. Liu, J. Kong, P. Hu, Z. Chen, M. Huang and X. Chen, Nanoscale, 2013, 5, 6430–6438 RSC.
- H. Fu, G. Yang, S. Gai, N. Niu, F. He, J. Xu and P. Yang, Dalton Trans., 2013, 42, 7863–7870 RSC.
- X. Teng, Y. Zhu, W. Wei, S. Wang, J. Huang, R. Naccache, W. Hu, A. I. Y. Tok, Y. Han, Q. Zhang, Q. Fan, W. Huang, J. A. Capobianco and L. Huang, J. Am. Chem. Soc., 2012, 134, 8340–8343 CrossRef CAS PubMed.
- X. Liang, X. Wang, J. Zhuang, Q. Peng and Y. Li, Adv. Funct. Mater., 2007, 17, 2757–2765 CrossRef CAS.
- N. C. Seeman, Nature, 2003, 421, 427–431 CrossRef PubMed.
- H. Qiu, G. Chen, L. Sun, S. Hao, G. Han and C. Yang, J. Mater. Chem., 2011, 21, 17202–17208 RSC.
- Y. Ding, X. Teng, H. Zhu, L. Wang, W. Pei, J.-J. Zhu, L. Huang and W. Huang, Nanoscale, 2013, 5, 11928–11932 RSC.
- L. Wang, H. Chen, D. Zhang, D. Zhao and W. Qin, Mater. Lett., 2011, 65, 504–506 CrossRef CAS PubMed.
- L. Wang, X. Xue, F. Shi, D. Zhao, D. Zhang, K. Zheng, G. Wang, C. He, R. Kim and W. Qin, Opt. Lett., 2009, 34, 2781–2783 CrossRef CAS PubMed.
- M. Pang, J. Feng, S. Song, Z. Wang and H. Zhang, CrystEngComm, 2013, 15, 6901–6904 RSC.
- S. Hao, L. Sun, G. Chen, H. Qiu, C. Xu, T. N. Soitah, Y. Sun and C. Yang, J. Alloys Compd., 2012, 522, 74–77 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: HRTEM images and EDS data of the NaScF4:Yb/Er and Na3ScF6:Yb/Er nanocrystals. TEM images of NaxScF3+x:Yb/Er nanocrystals synthesized at NaF:Ln3+ molar ratios of 14 and 20. XRD patterns of the NaxScF3+x:Yb/Er nanocrystals synthesized at NaF:Ln3+ molar ratios of 6, 8, 10, 14 and 20. XRD patterns of the NaYF4:Yb/Er and NaGdF4:Yb/Er nanocrystals synthesized at a NaF:Ln3+ molar ratio of 10. XRD and UC luminescence spectra of the corresponding NaYF4:Yb/Er, NaYbF4:Er and ultra-small Na3ScF6:Yb/Er nanocrystals. See DOI: 10.1039/c4nr06637e |
| This journal is © The Royal Society of Chemistry 2015 |