Clinoptilolite nano-particles modified with aspartic acid for removal of Cu(ii) from aqueous solutions: isotherms and kinetic aspects
Abstract
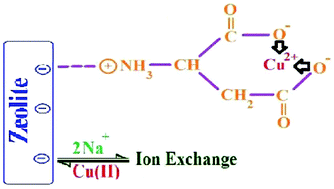
Raw and aspartic acid (ASP)-modified clinoptilolite (in both nano (NCP) and micronized (MCP) dimensions) were studied for their removal of heavy metal cations. The raw and modified samples were characterized by XRD, SEM, BET, TEM, FT-IR, TG and energy dispersive X-ray spectroscopy (EDX). Both raw and modified NCP showed better activity than the corresponding MCP for removal of the tested heavy metals, especially copper cations. The best activity was obtained by NCP modified in ASP solution at pH 6. The removal of Cu(II) ions increased during 300 min contact time. The kinetic isotherms showed that the adsorption kinetic could be well described by the pseudo-second-order rate equation. The Dubinin–Radushkevich and Langmuir isotherms indicated monolayer sorption of Cu(II) on the surface of the adsorbent via a chemical sorption phenomenon. The negative ΔG confirmed an exothermic and spontaneous removal process.

 Please wait while we load your content...
Please wait while we load your content...