Electrochemical reduction of CO2 to HCOOH using zinc and cobalt oxide as electrocatalysts
Abstract
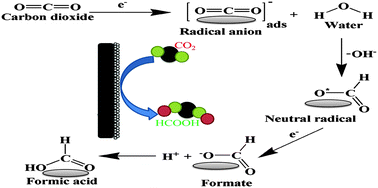
The present work studies the effect of a synthesized zinc (Zn) electrocatalyst towards the reduction of CO2 to products electrochemically (RCPE), using cobalt oxide (Co3O4) for water oxidation. The Zn catalyst was prepared using an electrodeposition method, with zinc chloride dihydrate (ZnCl2·2H2O) solution. The synthesized electrocatalyst was characterized by XRD, FTIR and particle size analysis, which confirmed the formation of Zn particles. Electrodes were prepared by depositing the synthesized Zn and Co3O4 on graphite plates, which were used as the cathode and the anode, respectively. The effects of applied voltage with time on the RCPE were studied in carbonates and bicarbonates of sodium and potassium electrolytes. Ultra-fast liquid chromatography (UFLC) was used for the detection of end products from the RCPE. However, HCOOH was the only product formed under all applied conditions. Maximum efficiencies were observed in bicarbonates, rather than carbonates. In KHCO3 electrolyte solution at 1.5 V, maximum Faradaic efficiencies of 78.54% and 78.46% for HCOOH were obtained after 5 and 10 min reaction. Similarly, in NaHCO3 solution, efficiencies of 60.5% and 64.7% after 5 min and 10 min duration were obtained at 2.5 V. The ability of the synthesized electrocatalyst towards the RCPE was explained for all the applied conditions in detail.

 Please wait while we load your content...
Please wait while we load your content...