The dual role of pnicogen as Lewis acid and base and the unexpected interplay between the pnicogen bond and coordination interaction in H3N⋯FH2X⋯MCN (X = P and As; M = Cu, Ag, and Au)†
Abstract
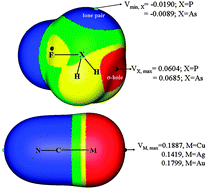
Ternary systems H3N⋯FH2X⋯MCN (X = P and As; M = Cu, Ag, and Au) as well as the corresponding pnicogen-bonded and coordination-bonded binary systems have been studied. The X atom acts as an electron acceptor and a donor in the pnicogen bond and coordination interaction, respectively, simultaneously playing both roles in the ternary complexes. Electrostatic interaction and charge transfer make dominant contributions to the stability of the pnicogen bond, while the coordination interaction results mainly from electrostatic and polarization interactions. Relativistic effects especially for the Au atom lead to some irregularity in interaction energy and the binding distance in the coordination interactions. In the ternary complex, the stronger coordination interaction strengthens the weaker pnicogen bond, while the pnicogen bond weakens the coordination interaction. The weakening of the coordination interaction was evidenced by the longer binding distance, lower electron density at the bond critical point, and smaller charge transfer. The change in the pnicogen bond and coordination interaction in the ternary complex has been rationalized with the analyses of the electrostatic potentials, occupancy on the lone pair of the X atom as well as the orbital interactions.

 Please wait while we load your content...
Please wait while we load your content...