What has fluorescent sensing told us about copper and brain malfunction?
Clara
Shen
and
Elizabeth J.
New
*
School of Chemistry, The University of Sydney, Building F11, NSW 2006, Australia. E-mail: elizabeth.new@sydney.edu.au; Tel: +61 2 9351 1993
First published on 19th November 2014
Abstract
There is growing evidence that copper and copper-binding proteins are common denominators in the mechanisms of neurodegenerative diseases such as Alzheimer's and Parkinson's. These pathologies have been linked to changes in copper homeostasis, but the question of whether this is a causal or effective relationship remains unanswered. A clearer understanding will require a way to visualise copper at a molecular level in vivo. Fluorescent metal sensing is one such tool, and a number of Cu(I) probes have been reported with excellent sensing properties and complementary studies that validate their biological application. This review critically evaluates the recent progress in fluorescent copper sensing and suggests some new directions for future study of copper neurochemistry.
The copper pools
Copper is an essential trace element that plays both catalytic and structural roles in proteins.1 In addition to the tightly-bound copper pool, comprising of copper bound to protein with very high binding affinity, the labile copper pool, while accounting for a much smaller proportion of total copper, is crucial to cellular health (Fig. 1).2 This is because proteins rely on rapid exchange of copper between chaperones and metalloproteins from low to high affinity, utilising an intermediate, labile pool. While there is almost no free copper within cells,3 the labile pool also includes weakly bound copper, and studies pertaining to the investigation of this pool will be the highlight of this review.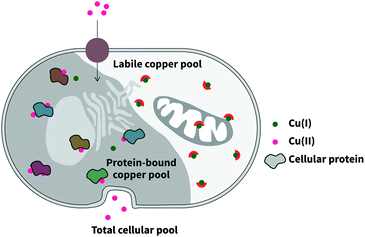 | ||
| Fig. 1 Intracellular copper comprises two distinct pools: the tightly-bound protein pool, and the labile pool of bioavailable copper. | ||
The labile metal pool, in particular, is sensitive to environmental changes such as pH, oxidative stress, and immunological stress.4 This is certainly the case for copper, and many inherited and progressive human disorders have been attributed both causatively and peripherally to perturbations in copper homeostasis. This ultimately speaks to the narrowness of its concentration window, and concomitantly the body's expenditures in energy and protein to regulate its distribution and concentration. Knowledge about the labile copper pool and its subcellular localisation and exchange kinetics is incomplete, and will require the use of some advanced sensing techniques and chemical tools. In particular, the study of the labile copper pool requires sensitivity to how tightly copper is bound, and to its oxidation state, rather than the total distribution of copper.5 This can be achieved by the use of fluorescent sensors,6 the focus of this review. This review will also primarily centre on the sensing of Cu(I), as this is the main form of labile copper in the reducing intracellular environment, but the study of intracellular labile Cu(II) and extracellular Cu(I) and Cu(II), beyond the scope of this review, could provide valuable complementary information.
Copper and disease
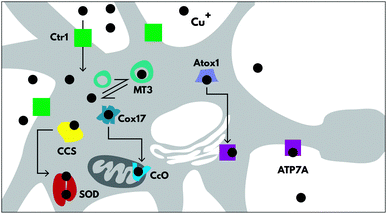
Copper trafficking and homeostasis are under tight control, especially in the brain, with specific proteins for copper uptake and export (CTR1 and ATP7A respectively), and for copper delivery to cuproenzymes (including CCS, Atox1 and Cox17) (Fig. 2), which are extensively reviewed elsewhere.7 In neurons, copper is also released from vesicles into the synapse, in a Ca2+-dependent process.8There is very little room for error in copper homeostasis, and misregulation can lead to serious consequences and even disease genesis. This can be attributed to two reasons; copper is essential to enzyme function, (e.g. COX1, Cu/Zn SOD, CP, SCO1, PrPc), so any changes to either the available copper pool or the ability of copper to bind and interact correctly with its hosts will have adverse cellular changes. Copper is also useful because, like iron, it is redox active and therefore plays a catalytic role in proteins.
Copper's redox activity is a liability to cellular health due to its ability to participate in Fenton chemistry and generate damaging free radicals.
Genetic diseases of copper
There are a number of genetic abnormalities that are associated with copper deficiency and excess, leading to severe pathological consequences. Two primary examples are Menkes and Wilson's disease, which are caused by mutations of copper export proteins ATP7A and ATP7B respectively.9,10 ATP7A is expressed in most cell types, and Menkes disease is manifested in copper accumulation in the small intestine and kidneys, and copper deficiency elsewhere, including in the brain. On the other hand, ATP7B is expressed primarily in the liver, and in Wilson's disease, the high concentrations of copper in the liver are released into the bloodstream, resulting in high copper levels throughout the body, including the brain. The neurological pathologies that accompany these diseases highlight the importance of tightly-regulated copper levels in the brain. A number of studies have elucidated the proteins involved and causative malfunctions, and investigations into their pathological mechanisms are underway in many research groups.11,12 This review will not focus on these diseases, but will instead examine recent progress in investigating copper and neurodegenerative diseases, where copper may play a causative role.Neurodegenerative diseases
Both copper deficiency and excess copper lead to altered brain function,7 highlighting the importance of studying the distribution and nature of copper within the brain, rather than just measuring total levels. A common factor in many neurodegenerative diseases is the presence of protein aggregation. There is strong evidence for copper-mediated aggregation of many different protein and protein fragments, which in turn cause common diseases as described in a number of comprehensive reviews.13–15Alzheimer's disease (AD) is a progressive neurodegenerative disease characterised by amyloid plaque formation16 and neurofibrillary tangles, which results in neuronal cell loss/dysfunction and eventual death. Amyloid plaques are formed when the Aβ peptide, a notoriously aggregative molecule produced by the proteolysis of amyloid precursor protein (APP), forms insoluble deposits in the brain. There is incomplete knowledge of how amyloid plaques form, and the mechanism by which Aβ aggregates. The neurotoxicity of plaques and neurofibrillary tangles is attributed primarily to reactive oxygen species (ROS)-mediated oxidative injury.17 A number of indicators of oxidative stress found in neurons have supported this claim, including nitration, advanced glycation, free carbonyls, and nucleic acid oxidation.18–20 Copper has been linked to a number of these mechanistic factors of AD;21 it is found in high concentrations at the site of amyloid plaques, it has been shown to interact with both APP and Aβ (with attomolar affinity),22,23 and may in fact catalyse the aggregation and precipitation of Aβ.24 Furthermore, both copper and iron are responsible for the generation of H2O2,25,26 which can then permeate tissue boundaries and react with reduced Fe and Cu, causing serious damage, with the production of the hydroxyl radical OH˙.27,28
Copper dysregulation has also been implicated in other neurodegenerative diseases, helpfully reviewed elsewhere.29,30 Parkinson's disease, which is caused by progressive loss of dopamine-producing cells in the substantia nigra, is associated with elevated copper of free copper in the cerebrospinal fluid,31 but depressed copper levels in various regions of the brain.32 Familial amyloid lateral sclerosis involves mutation of the Cu/Zn-superoxide dismutase, perturbing both copper levels within the cell, and decreasing the cell's ability to scavenge harmful reactive oxygen species.33,34 The term “prion diseases” describes the class of fatal diseases caused by the accumulation of misfolded PrPc (PrPSc),35 including Creutzfeld–Jacob disease and kuru. The healthy form binds to copper and exhibits antioxidant activity, but may also have roles in copper uptake and the targeted delivery of copper to specific proteins.36–38 The mechanism of conversion of PrPSc is unclear, but there is evidence to suggest that altered copper homeostasis plays a role.39,40 Finally, the rare autosomal-dominant Huntington's disease involves a defective form of the huntingtin protein, leading to cellular damage through various mechanisms including protein aggregation and oxidative stress.41 Copper may exacerbate the condition by forming a redox-active complex with huntingtin, promoting its aggregation.42
It is clear that in these diseases, the bioavailable, labile copper pool plays a significant role, as well as specific copper proteins. There is certainly a need for tools to address the many unanswered questions regarding the exact roles of copper in neurodegenerative disease, and to elucidate the forms and localisation of the most damaging copper pools.
Fluorescent metal ion sensing
Recently, there has been a shift to focussing on copper as a dynamic element in cellular processing, rather than merely as a static component of the proteome. Investigations into the molecular processes and regulation of copper have been led with the development of chemical tools and imaging techniques such as X-ray technology, small molecule fluorescent sensors, and genetically encoded protein sensors. While synchrotron X-ray fluorescence (SXRF) is currently a leading technique in quantitative metal imaging, and can provide information about the total metal pool, confocal microscopy, when used concurrently with responsive fluorophores, is most useful to studying the labile metal pools.43Fluorescence imaging is a cornerstone of chemical biology, and allows researchers to image cellular conditions at a molecular level.44 Research into fluorescent metal sensors has been progressing rapidly, giving rise to a large number of highly selective metal ion probes which have been used in cellulo to give quantifiable, spatially resolved images of metal concentration and distribution.44
Requirements for a useful fluorescent probe
Molecular probes have the ability to report on specific chemical conditions in a cell, but their design requires careful consideration. Large, measurable changes in fluorescence in response to an analyte with high optical brightness and intensity are desirable for microscopy experiments. High selectivity is required to provide an accurate result, and probes which change their emission wavelength on analyte interaction (so-called “ratiometric probes”) are ideal, as they enable internal standardisation of the probe's response by accounting for environmental variations in probe distribution and concentration.45Most reported Cu(I) sensors employ a fluorophore-receptor scaffold, which are usually based upon bis(2-((2-(ethylthio)ethyl)-thio)ethyl)amine (BETA) BETA is commonly attached to fluorophore scaffolds, as well as a number of its analogues (Scheme 1). BETA has a high affinity and selectivity for Cu(I) above other metal ions.46 Cu(I) is likely to have greatest interaction with the soft thioethers, while enhancing interactions with the nitrogen atom should maximise the impact of binding on the fluorophore's excited state.
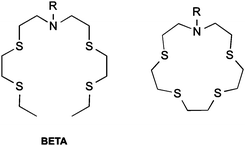 | ||
| Scheme 1 Receptors commonly employed in Cu(I) sensing. Left: BETA. Right: closed-ring form of BETA (crown-15-thioether). | ||
Challenges in sensing copper
There are a number of general challenges in fluorescent sensing, as well some specific to the sensing of copper. In general, fluorescent molecules tend to possess complex photophysics, and it is difficult to predict how analyte-binding will perturb the system, especially for novel fluorophores. The difficulty is enhanced in the case of fluorescence because molecular processes in the excited state can vary greatly from the ground state.47 Probe synthesis is often labour-intensive and non-facile, with low yields and purity, and within the field, syntheses tend to focus on individual (or small families of) probes, rather than utilising building blocks and divergent synthesis approaches to generate large libraries, as is the practice in medicinal chemistry. Finally, accurate characterisation of probes requires comparison to existing standards, which are often unavailable, particularly for investigation of new analytes.Despite the high demand and need for effective copper imaging tools, sensing and quantifying the labile copper pool in particular remains a challenge, for a number of reasons. Labile copper is present in extremely low concentrations in the body (approximately 10−13 M in blood plasma).3 Within cells, copper primarily exists protein-bound in the form of Cu(II), but intracellular free copper also interacts with a wide host of metallochaperones and transport proteins. The femtomolar to attomolar binding affinity for copper of some metallochaperones has been reported,48 which means that receptors of the labile pool must be able to sense sub-attomolar concentrations, and must therefore have correspondingly tight binding affinities. The rapid exchange kinetics of these metallochaperones further serves to compound this difficulty.49 The Fahrni group has reported a set of three ligands that form well-defined complexes with Cu(I), with known binding affinity.50 These ligands can be used to buffer Cu(I) concentrations, enabling determination of copper binding affinities of other ligands (including proteins). A final challenge in imaging copper is its tendency to act as an effective fluorescence quencher, mainly due to metal ligand charge transfer states which undergo rapid intersystem crossing to non-fluorescent triplet states.51
In assessing existing sensors and potential sensing strategies, it is essential to continually evaluate their chemical and biological properties. The true value of a chemical probe is not in the elegance of its fluorescence spectrum, or its ability to sense Cu(I) in a cuvette, and cannot even be evaluated by its ability to detect exogenously added Cu(I) to cells, but instead in its ability to report on changes in the labile copper pool in cells and systems in health and disease. A probe that meets this criterion would represent a truly significant contribution to the toolbox of copper biologists. This review will focus upon the biological studies and fluorescence properties of a number of monovalent copper probes, and identify the most promising strategies for future studies.
Biologically compatible Cu(I) probes
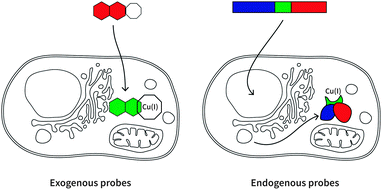
Here we will review a range of selective Cu(I) fluorescent sensors developed over the past decade, summarised in Table 1. Probes will be discussed according to their uses in cellular investigations; whether the visualisation of copper-supplemented cells, copper depleted cells, or stimuli-affected copper distribution in cells. Two types of fluorescent probes will be discussed: exogenous and endogenous probes (Fig. 3). The former are generally single-molecule species containing a known fluorophore, appended with a high affinity receptor for Cu(I), and in some cases with sub-cellular targeting groups (Scheme 2). The latter are genetically encoded probes prepared as recombinant proteins. Such sensors tend to be fluorescent proteins bearing a copper-binding group, which undergoes conformational changes upon interaction with Cu(I). Both types of probes have relative advantages and disadvantages, which will be explored in the following sections.| Cu(I) probe | Endogenous/exogenous | Cell type or animal model | Ex/Em filter (nm) | Binding affinity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KD) KD) |
Copper treatment or stimulus |
|---|---|---|---|---|---|
| a BCS = bathocuproine disulfonate. b TEMEA = tris(ethylthioethyl)amine. | |||||
| CTAP-152 | Exogenous | NIH 3T3 | 10.4 | ||
| CS153 | Exogenous | HEK293T | 543/560 | 11.4 | 100 μM CuCl2, 7 h |
| FluTPA254 | Exogenous | HeLa | 488/500–600 | N/A | 100 μM CuCl2, 8 h |
| Amt1-FRET55 | Endogenous | CHO-KT | 440/20, 535/485 | 17.1 | 100 nM CuSO4, 1 min |
| RCS156 | Exogenous | C6 | 488/506–720 | 10.4 | BCS,a TEMEAb |
| CS357 | Exogenous | HEK293T, primary rat hippocampal neurons | 530/540–700 | 13.1 | BCS, KCl, TEMEA, Dantrolene, Nifedipine |
| Mito-CS158 | Exogenous | HEK293T | 543/554–650 | 11.1 | 300 μM CuCl2, 18 h BCS |
| ACu159 | Exogenous | HeLa, rat hippocampal slices | 750/450–550TP (two photon) | 10.7 | 100 μM CuCl2, 7 h |
| Ace1-FRET60 | Endogenous | E. Coli | 433/453–600 | 17.3 | |
| Mac1-FRET60 | Endogenous | E. Coli | 433/453–600 | 19.01 | |
| CS790AM61 | Exogenous | HEK293T, SKH-1 mice, ATP7A−/− mice | 745/790, 760/800 | 10.5 | 100 μM CuCl2, 12 h |
| Probe 362 | Exogenous | MG63 | 750/792 | 11.2 | 200 μM CuCl2, 7 h |
| Napththyl-CS163 | Exogenous | SH-SY5Y | 405/440–510 | 100 μM CuSO4, 6 h | |
| 488/500–530 | |||||
| YAG464 | Endogenous | HeLa | 18.3 | 50 μM Cu(I), 0–5 min | |
| EGFP-145Amt165 | Endogenous | CHO | 18.3 | 1 mM CuSO4 | |
| OBEP-CS166 | Exogenous | SH-SY5Y | 543/550–605 | 13.4 | 100 μM CuSO4, 4 h |
| Ar-4167 | Endogenous | HEK293T | 550/580 | 17.9 | 1 mM Cu(II) |
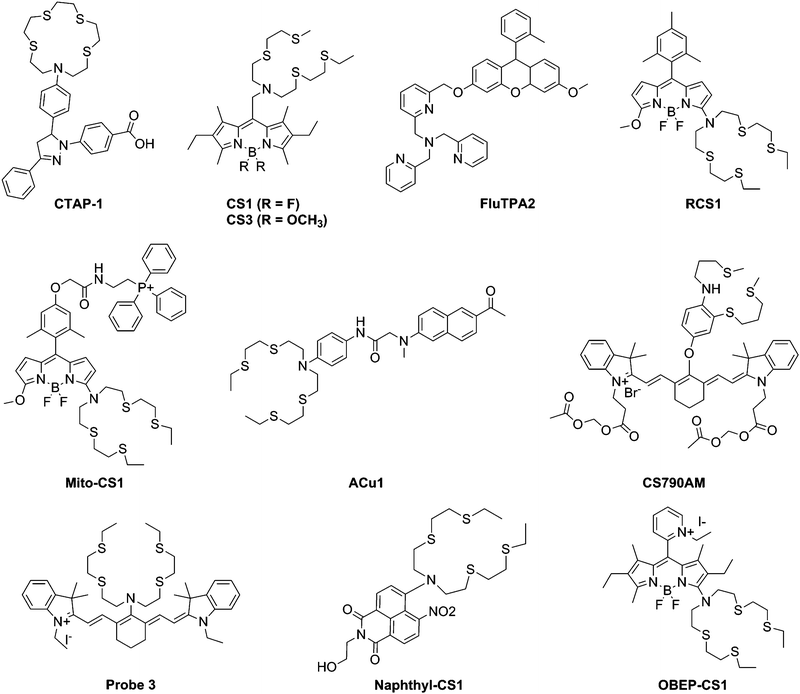 | ||
| Scheme 2 Structures of exogenous fluorescent Cu(I) probes mentioned in Table 1. | ||
Visualising supplemented copper in cells and animals
Copper supplementation of cells is a common way to demonstrate the applicability of a sensor for detecting copper levels, but in such studies, copper is often used at concentrations that are extremely disproportionate to physiological copper levels. Nevertheless, these preliminary studies are important to gauge a sensor's cellular uptake, localisation and copper response, and to confirm a positive response that is not affected by other biomolecules and conditions.The first generation of Cu(I) fluorescent sensors for cellular imaging was pioneered by Fahrni et al. with the introduction of CTAP-1, based on a pyrazoline fluorophore and a thioether-rich macrocyclic receptor.52 Shortly after, the Chang group reported a BODIPY-based fluorescent probe accessorised with a BETA receptor, CS1, which was able to detect copper in supplemented HEK293T cells.53 The general popularity of BODIPY-based biological probes can be attributed to their favourable emissive properties, high quantum yield, photostability, solubility and cell compatibility.68 Other BODIPY probes will be discussed later. Subsequent studies of CS1 demonstrated its utility in studying the endogenous copper pool, and in studies of neurons, which will be discussed below.
The Yamamoto group similarly presented a single molecule fluorescent probe for copper based on fluorescein with a TPA ligand, and showed turn-on fluorescence in copper-supplemented HeLa cells in the visible range.54 Unlike CTAP-1 and CS1, FluTPA reacts with Cu(I) irreversibly in a debenzylation reaction resulting in formation of the fluorescent form of fluorescein. Such reaction-based probes are able to capture transient events in the cell, but are unable to report on fluctuations of copper concentrations, or movement of copper within the cell over time.
Genetically encoded protein-based fluorescent sensors are popular in the field of molecular imaging, as they exhibit suitable biological properties such as tight copper binding affinity, selectivity and intracellular targeting due to the nature of their origin. However, there is always a risk that genetically expressed proteins will influence the conditions of the cell and especially copper distribution. Furthermore, the requirement to transfect the cell with a plasmid can restrict the cell types that can be readily interrogated by fluorescent protein-based sensors. The He group pioneered the field of genetically-encoded Cu(I) sensors, developing a FRET-based fluorescent Cu(I) sensor using a genetically encoded Amt1 group (a copper dependent regulator in yeast), flanked by cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP).55 This protein, Amt1-FRET, exhibited FRET-based fluorescence changes upon copper-binding-induced conformational changes to Amt1 in CHO-KT cells.
The He group subsequently developed fluorescent proteins Ace1-FRET and Mac1-FRET.60 These two proteins contained CFP and YFP tethered by different binding domains, and they therefore exhibited different Cu(I)-binding affinities. By constructing calibration curves for each probe with changing copper concentration, the researchers were able to determine the approximate concentration of copper within yeast, demonstrating the considerable promise of ratiometric fluorescent probes for quantitative determinations.
Wang et al. also recently reported a protein based Cu(I) sensor using green fluorescent protein with an inserted Amt1 domain, EGFP145Amt1.65 Rather than operating by a FRET interaction, it was designed in such a way that copper binding would induce structural distortion of the β-barrel conformation of GFP (which is responsible for its fluorescence), resulting in fluorescence quenching due to the geometric constraints. Desiring better optical properties such as a longer emission wavelength, the group went on to produce a library of red fluorescent protein-based Amt1 Cu(I) sensors, the most promising of which was Ar-41.67 By varying the insertion point of the Amt1 binder, they were able to fine-tune the brightness of the protein's fluorescence, which was switched off in the presence of four Cu(I) atoms. Indeed, the probe showed emission at 580 nm compared to 515 nm for EGFP145Amt1, but cellular experiments showed a slow response time (greater than 10 minutes) to cellular copper changes.
There have been great advances in the development of Cu(I) probes, but the true test of their applicability lies beyond their ability to sense copper supplementation.
Visualising the endogenous and depleted cellular copper pools
Fluorescent probes have the potential to provide spatial and temporal visualisation of the labile copper pools. A more powerful test of a probe's ability to sense these pools is its power to distinguish copper levels in resting cells from those in cells depleted of copper. Such sensitivity is a good indication that a probe will be useful in detecting physiological or pathological changes in copper levels. Subsequent to its initial publication, CS1 has been used in various biological studies, demonstrating its utility to study endogenous copper69,70 – its use in studying copper pools in neurons will be discussed in the following section.Reports of the development of more recent probes have included demonstration of probe sensitivity to basal and depleted copper levels. A survey of these probes highlights the varied advances that have been made in Cu(I) sensing. The Chang group followed up their first copper sensor with CS3,57 with a higher quantum yield and greater dynamic range than its precursor. They demonstrate the utility of CS3 in distinguishing the copper levels of control HEK293T cells with those depleted of copper by the addition of bathocuproine disulfonate (BCS: a copper chelator).
In the same year, Cho et al. published a two-photon fluorescent probe for Cu(I), ACu1,71 which contains a naphthalene-based fluorophore appended with the BETA receptor. Two-photon excitation at 750 nm showed clear differences between basal, supplemented and depleted copper levels, with a greater fold-increase in fluorescence than previously reported BODIPY probes. According to their co-localisation experiments with co-stains, ACu1 showed a high Pearson co-localisation coefficient with MitoTracker Red FM. Interestingly, the authors also performed two-photon imaging of the probe in rat hippocampal slices, showing a fluorescence penetration depth of 90–220 μM, demonstrating its utility beyond cultured cells. Fluorescence images of both untreated and copper-treated slices were taken and revealed some tissue-specific distribution of copper, with higher levels of Cu(I) in the CA1 and CA3 regions.
The Chang group further expanded the family of BODIPY-based Cu(I) sensors with Mito-CS1, a mitochondrial targeted reporter for labile Cu(I).58 In HEK293T cells, the probe was able to visualise both basal, excess and depleted copper levels, and colocalisation experiments with Rhodamine 123 confirmed its subcellular location. Further experiments in human fibroblasts measured a dynamic Cu(I) pool in mitochondria, and revealed that in cells with mutated copper chaperones SCO1 and SCO2, cells prioritised the maintenance of the mitochondrial copper pool over the cytoplasmic pool.
Recently, another BODIPY-based probe OBEP-CS1 developed by Sfrazzetto et al. was reported.66 The alkylpyridinium group directs the probe to the mitochondria, confirmed by colocalisation experiments using Mitotracker Deep Red. In SH-SY5Y cells, the probe showed a fluorescence decrease upon incubation in copper-containing medium. The probe is highly compatible with cells, showing minimal cytotoxicity, but the utility of this probe is limited by the fact that it is a turn-off probe – for such probes, it is impossible to distinguish regions of the cell in which there is no probe from regions of the cell in which both probe and copper are present.
Another significant achievement in the development of Cu(I) probes has been in the area of ratiometric sensing, where the emission spectra exhibit two or more emission peaks, which respond differently to the addition of Cu(I). Towards this end, the Chang group reported the BODIPY-based RCS1,61 in which the higher energy emission peak is insensitive to Cu(I) concentration, while the lower energy peak increases in intensity with Cu(I). This probe was used to observe changes in the endogenous copper pool in HEK293T cells in response to ascorbate treatment. In 2013, Satriano et al. reported another ratiometric, cell-compatible probe for Cu(I) based on a naphthalimide fluorophore with the BETA receptor.63 This probe was used to observe changes in basal copper levels in neuroblastoma SH-SY5H cells through a change in blue/green fluorescence ratio.
Near infrared probes are gaining popularity due to some superior properties, such as their much less damaging excitation wavelengths and the penetration depth provided by infrared range emissions. Probe 3 is a symmetrical cyanine-based fluorescent probe for Cu(I) containing a BETA receptor reported by the Wan group.62 Fluorescence images of the probe in MG63 cells showed both supplemented copper levels as well as the ascorbic acid-triggered release of the endogenous copper pools, with a turn on in fluorescence at approximately 800 nm. Further treatment with BETA alone showed subsequent diminished fluorescence.
Further advances were made in near-IR imaging when Chang et al. reported the use of a novel infrared imaging agent CS790AM in murine Wilson's disease models.61 This is the first reported use of a Cu(I) probe in live animal imaging, and was able to reveal fluctuations of Cu(I) in mice after supplementation with CuCl2. Furthermore, ATP7B-deficient mice were imaged with CS790AM and shown to have elevated Cu(I) by measuring the intensity of fluorescence emission at 800 nm. This is in agreement with the fact that ATP7B is responsible for copper export in some tissue types. Dynamic imaging of Cu(I) levels was achieved after treatment with a copper chelator was correlated with a decrease in fluorescence intensity, and confirmed with ICP-MS of tissue extracts.
This survey reveals that the past five years has yielded a large toolbox of probes with demonstrated utility in detecting changes in the endogenous labile copper pool. These probes exhibit valuable properties for potential use in understanding copper in the brain: reversibility, the ability to control localisation (although, at this stage, only to mitochondria and the lysosome), ratiometricity, and near-IR emission. Given the promise of these probes, it is important to now consider how they have found use in studies of brain copper, and to evaluate whether they have met their potential.
Studying copper distribution in neurons
Despite the promise of copper imaging, the number of studies using fluorescent Cu(I) probes in studies of brain cells is relatively few. Notably, a large proportion of probes have not been utilised in reported biological studies beyond the initial publication. CS1 has been used in neuronal studies, with mixed success: while one study reported that the relatively weak binding of CS1, and its lysosomal localisation, rendered it insensitive even to exogenously-added copper in neurons and glia,72 another study successfully applied the probe to the investigation of copper levels in brain tissue of patients with and without cerebral amyloid angiopathy.73 These findings highlight the importance of matching the probe to the cell type and sub-cellular pool being investigated.Perhaps most significantly, Chang et al. used CS3 to investigate copper distribution upon neuronal depolarisation using both metal chelators and other chemical reagents,57 showing that there was a calcium-dependent redistribution of Cu(I) from the cell body to the processes upon KCl-induced depolarisation. This could potentially be extended in the future to studying the movement of Cu(I) in diseased states of neuronal cells, and confirms that these probes can be useful in cells of this type.
Similarly, Urso et al. performed experiments on neuroblastoma cell models of prion disease, using Phen Green Sk to visualise Cu transport throughout the cell and its relationship with PrPc protein.74 The choice of fluorophore is interesting, given that Phen Green SK shows no selectivity for Cu over Fe, and that the labile iron pool has a far higher concentration (μM) than labile copper.75 Nevertheless, the findings of the study were interpreted to signify that PrPc, which can bind up to 4 Cu atoms,36,37 may drive the delivery of Cu across the cell membrane, and loss of PrPc function leads to dysregulation of Cu(I). Cu(II) deprived cells showed increased PrPc activity, and concomitantly, the removal of PrPc led to a decrease in copper influx.
Investigating copper-based therapeutics
Fluorescence sensing is not restricted to the visualisation of changes in endogenous cellular metal pools, and may provide insight into the mechanism and pharmacokinetics of medicinally relevant metal complexes. Recently, a number of encouraging Cu(II) and Zn(II) based-therapeutic compounds have been reported, including copper(II) bis(thiosemicarbazonato) complexes (Cu(II)(atsm) and Cu(II)(gtsm)),76 and have been shown to have neuroprotective77,78 and anti-cancer properties.79,80 These complexes are non-fluorescent, but researchers have used fluorescently tagged derivatives to circumvent this problem and investigate the uptake, mechanism and biodistribution of these drugs. Fluorescent pyrene-appended derivatives of Cu(II) (atsm) and Cu(II)(gtsm) were developed by the Donnelly group,81 and with collaborators, they were able to track its uptake into HeLa cells by confocal microscopy, which was confirmed with ICP-MS. Cells treated with the tagged Cu(II) complex showed it localised primarily in the cytosol and lysosomes. The pyrene complex was also used to investigate the neuroprotective action of Cu(II)(atsm) in neuronal (M17) and glial (U87MG) cells.82Future directions for copper sensing
This survey of fluorescent Cu(I) sensors has revealed a large set of probes with demonstrated biological utility, that have yet to be used to greatest effect in the understanding brain copper biology. Of these probes, ratiometric probes show particular promise for the study of cultured brain cells. They have the ability to eliminate effects of local probe concentration. Importantly, for the study of brain copper, it will be valuable to observe the movement of copper within cells, or even between cells, in response to stimuli. Use of turn-on (intensity-based) probes for such studies is confounded by the fact that probe distribution is unlikely to be uniform throughout the cell, but the use of ratiometric probes will make this task much easier. Furthermore, the reported near-IR copper probes, CS790AM61 and Probe 3,62 are likely to be particularly valuable for the study of brain slices, or even for use in imaging the brains of small live animals, such as mice. It is important to note, however, that a prevailing question remains in the general use of fluorescent sensors; that of whether the probe is perturbing the system it seeks to study. A definitive method to answer this question, showing that exogenous probes sense but do not perturb metal homeostasis in the cell, would be a most valuable contribution to this field.78Despite the untapped promise of existing probes, there are a number of areas that require further research. Copper sensing has made great advances, but the field still lags behind the sensing of other metals such as iron and zinc, particularly in areas of quantification, whole animal imaging and investigation of the labile pool. A number of areas need to be improved and the properties overall of copper sensors need to be optimised for future biological application.
There is no doubt that existing probes could be used to better effect, but further design of Cu(I) sensors is certainly needed, in a number of key directions. Given the promise of both ratiometric and near-IR emitting probes, sensors that combine these two properties would have great advantage, as would pushing probe emission further into the infra-red to further enhance tissue penetration. Furthermore, while mitochondrial-targeted probes have been developed, there is a distinct lack of probes localised elsewhere within the cell – a set of probes targeted to each key organelle would enable understanding of the regulation of copper levels within these locations.
Quantifying metal concentrations within cells is difficult, especially for the labile copper pool. One possible solution is the use of multiple receptors in combination with ratiometric fluorophores, a method that has already been successfully employed using fluorescent proteins to determine yeast labile copper concentrations as discussed earlier,60 and using the absorbance of a variety of copper chelators to determine protein binding affinities.83 Understanding of brain copper biology will be greatly advanced by clear measurements of concentrations of labile metal (extracellular Cu(I) and Cu(II), as well as intracellular levels).
The advances in fluorescent copper reporting reviewed here come with a requirement to begin building a clearer picture of copper's role in the brain. Essential to this pursuit is the ability to simultaneously visualise multiple copper pools, so far typified by the elegant studies of Fahrni52 and Chang57 using fluorescent probes and synchrotron X-ray fluorescence. The delicate handling of copper is sensitive to physiological stress, potentially evoking opposing responses from the total, protein-bound, and labile copper pool. Monitoring these pools, in conjunction with the expression of key cuproproteins, will therefore be essential for investigating copper in the brain. Fortunately, techniques to study copper proteins and the total copper pool are well established, and in combination with the ongoing research of labile copper sensors, there is a promising future for this field.
Conclusions
In this review, we have covered a range of fluorescent exogenous and endogenous Cu(I) sensors, focussing on their application to biological studies. The utilisation of these probes, however, was found to fall short of the expectations of fluorescent imaging and its power in modern research. While the probes reported here were found to be highly selective, sensitive and versatile, cellular studies were generally limited to the visualisation of supplemented Cu(I), and even fewer studies applied sensors to neuronal systems, despite overwhelming evidence for the role of copper in neurodegeneration. The extremely small labile Cu pool and its movement in biological systems is certainly worthy of greater attention, and the strategies and techniques shown here to harness the power of fluorescent metal sensing will further our understanding of its role in the brain.Notes and references
- M. Olivares and R. Uauy, Am. J. Clin. Nutr., 1996, 63, S791–S796 Search PubMed.
- L. Yang, R. McRae, M. M. Henary, R. Patel, B. Lai, S. Vogt and C. J. Fahrni, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 11179–11184 CrossRef CAS PubMed.
- T. D. Rae, P. J. Schmidt, R. A. Pufahl, V. C. Culotta and T. V. O'Halloran, Science, 1999, 284, 805–808 CrossRef CAS.
- L. A. Finney and T. V. O'Halloran, Science, 2003, 300, 931–936 CrossRef CAS PubMed.
- E. J. New, Dalton Trans., 2013, 42, 3210–3219 RSC.
- D. W. Domaille, E. L. Que and C. J. Chang, Nat. Chem. Biol., 2008, 4, 168–175 CrossRef CAS PubMed.
- I. F. Scheiber, J. F. B. Mercer and R. Dringen, Prog. Neurobiol., 2014, 116, 33–57 CrossRef CAS PubMed.
- E. D. Gaier, B. A. Eipper and R. E. Mains, J. Neurosci. Res., 2013, 91, 2–19 CAS.
- P. C. Bull, G. R. Thomas, J. M. Rommens, J. R. Forbes and D. W. Cox, Nat. Genet., 1993, 5, 327–337 CrossRef CAS PubMed.
- S. Lutsenko, K. Petrukhin, M. J. Cooper, C. T. Gilliam and J. H. Kaplan, J. Biol. Chem., 1997, 272, 18939–18944 CrossRef CAS PubMed.
- H. Kodama, C. Fujisawa and W. Bhadhprasit, Curr. Drug Metab., 2012, 13, 237–250 CrossRef CAS.
- S. G. Kaler, Handb. Clin. Neurol., 2013, 113, 1745–1754 Search PubMed.
- A. I. Bush, Trends Neurosci., 2003, 26, 207–214 CrossRef CAS.
- C. A. Ross and M. A. Poirier, Nat. Med., 2004, 10, S10–S17 CrossRef PubMed.
- R. M. Llanos and J. F. B. Mercer, DNA Cell Biol., 2002, 21, 259–270 CrossRef CAS PubMed.
- C. L. Masters, G. Simms, N. A. Weinman, G. Multhaup, B. L. McDonald and K. Beyreuther, Proc. Natl. Acad. Sci. U. S. A., 1985, 82, 4245–4249 CrossRef CAS.
- L. M. Sayre, D. A. Zelasko, P. L. Harris, G. Perry, R. G. Salomon and M. A. Smith, J. Neurochem., 1997, 68, 2092–2097 CrossRef CAS.
- D. G. Smith, R. Cappai and K. J. Barnham, Biochim. Biophys. Acta, 2007, 1768, 1976–1990 CrossRef CAS PubMed.
- N. Ahmed, U. Ahmed, P. J. Thornalley, K. Hager, G. Fleischer and G. Münch, J. Neurochem., 2005, 92, 255–263 CrossRef CAS PubMed.
- B. Halliwell, J. Neurochem., 2006, 97, 1634–1658 CrossRef CAS PubMed.
- D. J. Waggoner, T. B. Bartnikas and J. D. Gitlin, Neurobiol. Dis., 1999, 6, 221–230 CrossRef CAS PubMed.
- C. S. Atwood, R. C. Scarpa, X. Huang, R. D. Moir, W. D. Jones, D. P. Fairlie, R. E. Tanzi and A. I. Bush, J. Neurochem., 2000, 75, 1219–1233 CrossRef CAS.
- F. Stellato, G. Menestrina, M. D. Serra, C. Potrich, R. Tomazzolli, W. Meyer-Klaucke and S. Morante, Eur. Biophys. J., 2006, 35, 340–351 CrossRef CAS PubMed.
- C. S. Atwood, R. D. Moir, X. D. Huang, R. C. Scarpa, N. Bacarra, D. M. Romano, M. K. Hartshorn, R. E. Tanzi and A. I. Bush, J. Biol. Chem., 1998, 273, 12817–12826 CrossRef CAS PubMed.
- X. Huang, M. P. Cuajungco, C. S. Atwood, M. A. Hartshorn, J. D. A. Tyndall, G. R. Hanson, K. C. Stokes, M. Leopold, G. Multhaup, L. E. Goldstein, R. C. Scarpa, A. J. Saunders, J. Lim, R. D. Moir, C. Glabe, E. F. Bowden, C. L. Masters, D. P. Fairlie, R. E. Tanzi and A. I. Bush, J. Biol. Chem., 1999, 274, 37111–37116 CrossRef CAS PubMed.
- X. Huang, C. S. Atwood, M. A. Hartshorn, G. Multhaup, L. E. Goldstein, R. C. Scarpa, M. P. Cuajungco, D. N. Gray, J. Lim, R. D. Moir, R. E. Tanzi and A. I. Bush, Biochemistry, 1999, 38, 7609–7616 CrossRef CAS PubMed.
- L. M. Sayre, G. Perry, P. L. Harris, Y. Liu, K. A. Schubert and M. A. Smith, J. Neurochem., 2000, 74, 270–279 CrossRef CAS.
- A. R. White, G. Multhaup, F. Maher, S. Bellingham, J. Camakaris, H. Zheng, A. I. Bush, K. Beyreuther, C. L. Masters and R. Cappai, J. Neurosci., 1999, 19, 9170–9179 CAS.
- E. Tiffany-Castiglioni, S. Hong and Y. Qian, Int. J. Dev. Neurosci., 2011, 29, 811–818 CrossRef CAS PubMed.
- I. F. Scheiber, J. F. B. Mercer and R. Dringen, Prog. Neurobiol., 2014, 116, 33–57 CrossRef CAS PubMed.
- M.-C. Boll, M. Alcaraz-Zubeldia, S. Montes and C. Ríos, Neurochem. Res., 2008, 33, 1717–1723 CrossRef CAS PubMed.
- D. A. Loeffler, P. A. LeWitt, P. L. Juneau, A. A. Sima, H. U. Nguyen, A. J. DeMaggio, C. M. Brickman, G. J. Brewer, R. D. Dick, M. D. Troyer and L. Kanaley, Brain Res., 1996, 738, 265–274 CrossRef CAS.
- D. R. Rosen, A. C. Bowling, D. Patterson, T. B. Usdin, P. Sapp, E. Mezey, D. McKenna-Yasek, J. O'Regan, Z. Rahmani, R. J. Ferrante, M. J. Brownstein, N. W. Kowall, M. F. Beal, H. R. Horvitz, J. Robert and H. Brown, Hum. Mol. Genet., 1994, 3, 981–987 CrossRef CAS PubMed.
- T. J. Lyons, A. Nersissian, H. Huang, H. Yeom, C. R. Nishida, J. A. Graden, E. B. Gralla and J. S. Valentine, JBIC, J. Biol. Inorg. Chem., 2000, 5, 189–203 CrossRef CAS.
- S. B. Prusiner, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 13363–13383 CrossRef CAS.
- P. C. Pauly and D. A. Harris, J. Biol. Chem., 1998, 273, 33107–33110 CrossRef CAS PubMed.
- E. Quaglio, R. Chiesa and D. A. Harris, J. Biol. Chem., 2001, 276, 11432–11438 CrossRef CAS PubMed.
- P. C. Wong, D. Waggoner, J. R. Subramaniam, L. Tessarollo, T. B. Bartnikas, V. C. Culotta, D. L. Price, J. Rothstein and J. D. Gitlin, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 2886–2891 CrossRef CAS PubMed.
- S. P. Leach, M. D. Salman and D. Hamar, Anim. Health Res. Rev., 2006, 7, 97–105 CrossRef PubMed.
- D. Wu, W. Zhang, Q. Luo, K. Luo, L. Huang, W. Wang, T. Huang, R. Chen, Y. Lin, D. Pang and G. Xiao, J. Cell. Biochem., 2010, 111, 627–633 CrossRef CAS PubMed.
- K. N. McFarland and J.-H. J. Cha, Handb. Clin. Neurol., 2011, 100, 25–81 Search PubMed.
- J. H. Fox, J. A. Kama, G. Lieberman, R. Chopra, K. Dorsey, V. Chopra, I. Volitakis, R. A. Cherny, A. I. Bush and S. Hersch, PLoS One, 2007, 2, e334 Search PubMed.
- M. Ralle and S. Lutsenko, BioMetals, 2009, 22, 197–205 CrossRef CAS PubMed.
- K. P. Carter, A. M. Young and A. E. Palmer, Chem. Rev., 2014, 114, 4564–4601 CrossRef CAS PubMed.
- G. Grynkiewicz, M. Poenie and R. Y. Tsien, J. Biol. Chem., 1985, 260, 3440–3450 CAS.
- M. M. Bernardo, M. J. Heeg, R. R. Schroeder, L. A. Ochrymowycz and D. B. Rorabacher, Inorg. Chem., 1992, 31, 191–198 CrossRef CAS.
- N. S. Finney, Curr. Opin. Chem. Biol., 2006, 10, 238–245 CrossRef CAS PubMed.
- Z. Xiao, J. Brose, S. Schimo, S. M. Ackland, S. La Fontaine and A. G. Wedd, J. Biol. Chem., 2011, 286, 11047–11055 CrossRef CAS PubMed.
- J. J. Benítez, A. M. Keller, D. L. Huffman, L. A. Yatsunyk, A. C. Rosenzweig and P. Chen, Faraday Discuss., 2011, 148, 71–82 RSC.
- P. Bagchi, M. T. Morgan, J. Bacsa and C. J. Fahrni, J. Am. Chem. Soc., 2013, 135, 18549–18559 CrossRef CAS PubMed.
- C. J. Fahrni, Curr. Opin. Chem. Biol., 2013, 17, 656–662 CrossRef CAS PubMed.
- L. Yang, R. McRae, M. M. Henary, R. Patel, B. Lai, S. Vogt and C. J. Fahrni, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 11179–11184 CrossRef CAS PubMed.
- L. Zeng, E. W. Miller, A. Pralle, E. Y. Isacoff and C. J. Chang, J. Am. Chem. Soc., 2006, 128, 10–11 CrossRef CAS PubMed.
- M. Taki, S. Iyoshi, A. Ojida, I. Hamachi and Y. Yamamoto, J. Am. Chem. Soc., 2010, 132, 5938–5939 CrossRef CAS PubMed.
- S. V. Wegner, H. Arslan, M. Sunbul, J. Yin and C. He, J. Am. Chem. Soc., 2010, 132, 2567–2569 CrossRef CAS PubMed.
- D. W. Domaille, L. Zeng and C. J. Chang, J. Am. Chem. Soc., 2010, 132, 1194–1195 CrossRef CAS PubMed.
- S. C. Dodani, D. W. Domaille, C. I. Nam, E. W. Miller, L. A. Finney, S. Vogt and C. J. Chang, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 5980–5985 CrossRef CAS PubMed.
- S. C. Dodani, S. C. Leary, P. A. Cobine, D. R. Winge and C. J. Chang, J. Am. Chem. Soc., 2011, 133, 8606–8616 CrossRef CAS PubMed.
- C. S. Lim, J. H. Han, C. W. Kim, M. Y. Kang, D. W. Kang and B. R. Cho, Chem. Commun., 2011, 47, 7146–7148 RSC.
- S. V. Wegner, F. Sun, N. Hernandez and C. He, Chem. Commun., 2011, 47, 2571–2573 RSC.
- T. Hirayama, G. C. Van de Bittner, L. W. Gray, S. Lutsenko and C. J. Chang, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 2228–2233 CrossRef CAS PubMed.
- X. Cao, W. Lin and W. Wan, Chem. Commun., 2012, 48, 6247–6249 RSC.
- C. Satriano, G. T. Sfrazzetto, M. E. Amato, F. P. Ballistreri, A. Copani, M. L. Giuffrida, G. Grasso, A. Pappalardo, E. Rizzarelli, G. A. Tomaselli and R. M. Toscano, Chem. Commun., 2013, 49, 5565–5567 RSC.
- J. Liu, J. Karpus, S. V. Wegner, P. R. Chen and C. He, J. Am. Chem. Soc., 2013, 135, 3144–3149 CrossRef CAS PubMed.
- J. Liang, M. Qin, R. Xu, X. Gao, Y. Shen, Q. Xu, Y. Cao and W. Wang, Chem. Commun., 2012, 48, 3890–3892 RSC.
- M. L. Giuffrida, E. Rizzarelli, G. A. Tomaselli, C. Satriano and G. Trusso Sfrazzetto, Chem. Commun., 2014, 50, 9835–9838 RSC.
- J. Liang, L. Guo, Y. Ding, L. Xia, Y. Shen, M. Qin, Q. Xu, Y. Cao and W. Wang, Biochem. Biophys. Res. Commun., 2014, 443, 894–898 CrossRef CAS PubMed.
- G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184–1201 CrossRef CAS PubMed.
- J. Beaudoin, R. Ioannoni, L. Lopez-Maury, J. Bahler, S. Ait-Mohand, B. Guerin, S. C. Dodani, C. J. Chang and S. Labbe, J. Biol. Chem., 2011, 286, 34356–34372 CrossRef CAS PubMed.
- D. Quaranta, T. Krans, C. Espírito Santo, C. G. Elowsky, D. W. Domaille, C. J. Chang and G. Grass, Appl. Environ. Microbiol., 2011, 77, 416–426 CrossRef CAS PubMed.
- C. S. Lim, J. H. Han, C. W. Kim, M. Y. Kang, D. W. Kang and B. R. Cho, Chem. Commun., 2011, 47, 7146 RSC.
- K. A. Price, J. L. Hickey, Z. Xiao, A. G. Wedd, S. A. James, J. R. Liddell, P. J. Crouch, A. R. White and P. S. Donnelly, Chem. Sci., 2012, 3, 2748–2759 RSC.
- M. Schrag, A. Crofton, M. Zabel, A. Jiffry, D. Kirsch, A. Dickson, X. W. Mao, H. V. Vinters, D. W. Domaille, C. J. Chang and W. Kirsch, J. Alzheimer's Dis., 2011, 24, 137–149 CAS.
- E. Urso, A. Rizzello, R. Acierno, M. G. Lionetto, B. Salvato, C. Storelli and M. Maffia, J. Membr. Biol., 2010, 233, 13–21 CrossRef CAS PubMed.
- S. Epsztejn, O. Kakhlon, H. Glickstein, W. Breuer and I. Cabantchik, Anal. Biochem., 1997, 248, 31–40 CrossRef CAS.
- B. M. Paterson and P. S. Donnelly, Chem. Soc. Rev., 2011, 40, 3005–3018 RSC.
- P. S. Donnelly, A. Caragounis, T. Du, K. M. Laughton, I. Volitakis, R. A. Cherny, R. A. Sharples, A. F. Hill, Q.-X. Li, C. L. Masters, K. J. Barnham and A. R. White, J. Biol. Chem., 2008, 283, 4568–4577 CrossRef CAS PubMed.
- P. J. Crouch, L. W. Hung, P. A. Adlard, M. Cortes, V. Lal, G. Filiz, K. A. Perez, M. Nurjono, A. Caragounis, T. Du, K. Laughton, I. Volitakis, A. I. Bush, Q.-X. Li, C. L. Masters, R. Cappai, R. A. Cherny, P. S. Donnelly, A. R. White and K. J. Barnham, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 381–386 CrossRef CAS PubMed.
- J. G. Cappuccino, S. Banks, G. Brown, M. George and G. S. Tarnowski, Cancer Res., 1967, 27, 968–973 CAS.
- B. K. Bhuyan and T. Betz, Cancer Res., 1968, 28, 758–763 CAS.
- S. Lim, K. A. Price, S.-F. Chong, B. M. Paterson, A. Caragounis, K. J. Barnham, P. J. Crouch, J. M. Peach, J. R. Dilworth, A. R. White and P. S. Donnelly, JBIC, J. Biol. Inorg. Chem., 2010, 15, 225–235 CrossRef CAS PubMed.
- K. A. Price, P. J. Crouch, S. Lim, B. M. Paterson, J. R. Liddell, P. S. Donnelly and A. R. White, Metallomics, 2011, 3, 1280–1290 RSC.
- Z. Xiao, L. Gottschlich, R. van der Meulen, S. R. Udagedara and A. G. Wedd, Metallomics, 2013, 5, 501–513 RSC.
| This journal is © The Royal Society of Chemistry 2015 |