Sequential myosin phosphorylation activates tarantula thick filament via a disorder–order transition†
Abstract
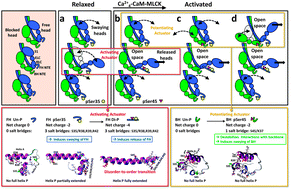
Phosphorylation of myosin regulatory light chain (RLC) N-terminal extension (NTE) activates myosin in thick filaments. RLC phosphorylation plays a primary regulatory role in smooth muscles and a secondary (modulatory) role in striated muscles, which is regulated by Ca2+via TnC/TM on the thin filament. Tarantula striated muscle exhibits both regulatory systems: one switches on/off contraction through thin filament regulation, and another through PKC constitutively Ser35 phosphorylated swaying free heads in the thick filaments that produces quick force on twitches regulated from 0 to 50% and modulation is accomplished recruiting additional force-potentiating free and blocked heads via Ca2+4-CaM-MLCK Ser45 phosphorylation. We have used microsecond molecular dynamics (MD) simulations of tarantula RLC NTE to understand the structural basis for phosphorylation-based regulation in tarantula thick filament activation. Trajectory analysis revealed that an inter-domain salt bridge network (R39/E58,E61) facilitates the formation of a stable helix–coil–helix (HCH) motif formed by helices P and A in the unphosphorylated NTE of both myosin heads. Phosphorylation of the blocked head on Ser45 does not induce any substantial structural changes. However, phosphorylation of the free head on Ser35 disrupts this salt bridge network and induces a partial extension of helix P along RLC helix A. While not directly participating in the HCH folding, phosphorylation of Ser35 unlocks a compact structure and allows the NTE to spontaneously undergo coil–helix transitions. The modest structural change induced by the subsequent Ser45 diphosphorylation monophosphorylated Ser35 free head facilitates full helix P extension into a single structurally stable α-helix through a network of intra-domain salt bridges (pS35/R38,R39,R42). We conclude that tarantula thick filament activation is controlled by sequential Ser35–Ser45 phosphorylation via a conserved disorder-to-order transition.
- This article is part of the themed collection: Chemical Biology in Molecular BioSystems


 Please wait while we load your content...
Please wait while we load your content...