Cyanobacteria as photosynthetic biocatalysts: a systems biology perspective†
Steinn
Gudmundsson
a and
Juan
Nogales
*b
aCenter for Systems Biology, University of Iceland, Sturlugata 8, 101 Reykjavik, Iceland
bDepartment of Environmental Biology, Centro de Investigaciones Biológicas-CSIC, Ramiro de Maeztu 9, 28040 Madrid, Spain. E-mail: jnogales@cib.csic.es
First published on 3rd November 2014
Abstract
The increasing need to replace oil-based products and to address global climate change concerns has triggered considerable interest in photosynthetic microorganisms. Cyanobacteria, in particular, have great potential as biocatalysts for fuels and fine-chemicals. During the last few years the biotechnological applications of cyanobacteria have experienced an unprecedented increase and the use of these photosynthetic organisms for chemical production is becoming a tangible reality. However, the field is still immature and many concerns about the economic feasibility of the biotechnological potential of cyanobacteria remain. In this review we describe recent successes in biofuel and fine-chemical production using cyanobacteria. We discuss the role of the photosynthetic metabolism and highlight the need for systems-level metabolic optimization in order to achieve the true potential of cyanobacterial biocatalysts.
Steinn Gudmundsson is an assistant professor in Computer Science at the University of Iceland. His research focuses on constraint based models of metabolic networks, algorithm development and the applications of such models in basic science and engineering. He studied Engineering at the University of Iceland and the Technical University of Denmark. He received his PhD in computer science from the University of Iceland for work in machine learning and time series analysis. During a brief stint as a postdoc at the Center for Systems Biology at the University of Iceland he became interested in systems biology and has been working on models of phototrophic organisms and the human metabolism since. |
Juan Nogales is a postdoctoral researcher in the Department of Environmental Biology at CIB-CSIC in Madrid (Spain). His research focuses on the study of the microbial metabolism at the systems level and its biotechnological implications. He studied Biology and Biochemistry at the University of Extremadura and received his PhD in Biochemistry and Molecular Biology focusing on microbial biodegradation from the University Complutense of Madrid (Spain). His postdoc training was performed at University of Iceland (Iceland) and University of California, San Diego (USA), where he delved into systems biology approaches to whole understanding of phototrophic metabolism and its biotechnological potential. |
Introduction
The recycling of CO2 into usable fuels, chemical building blocks and fine chemicals by photosynthetic organisms has received considerable interest in recent years due to the ever-increasing demand for energy, the depletion of fossil fuels and climate change. First generation biodiesel and chemicals derived from crops and biomass are increasingly being questioned over concerns such as high production costs and the competition with edible crops over land use.1,2 Microalgae are alternative sources of biodiesel and selected chemicals such as carotenoids; however, the costs of biomass harvesting and downstream processing are still far too high to make them economically feasible sources of fuels.3 In an attempt to reduce the costs, a new direct approach has been proposed. This approach uses photosynthetic organisms such as eukaryotic microalgae and cyanobacteria which have been engineered to convert CO2 directly into biofuel or high-value chemicals, without the need to synthesize and process high amounts of biomass. This approach implies continuous production and secretion of target metabolites from the culture while minimizing the production of biomass and undesirable byproducts.Cyanobacteria have been used as food sources and biofertilizers for centuries;4 they produce a broad spectrum of high-value compounds,5 have minimal nutritional requirements,6 and higher photosynthetic efficiency in terms of sunlight conversion into biomass and growth rates than all other photosynthetic organisms.7–9 These properties have led to great interest in using cyanobacteria as photobiocatalysts for chemical production. In addition, their primary metabolic capabilities have been modeled at the genome-scale,10–14 enabling quantitative predictions of cellular behavior, and they are amenable to genetic manipulation.15 Building upon these features and establishing a proof of concept of the direct production of chemicals from oxygenic photosynthesis, cyanobacteria have been successfully engineered to produce high value and biofuel-like compounds in the last few years (Table S1, ESI†). However, only a limited number of chemicals have been produced so far and the cyanobacterial biocatalysts are currently hampered by low yields, which prevents their application on a large scale. Overcoming these limitations requires a holistic strategy, which includes increasing the photosynthetic and CO2 fixation efficiencies, optimizing photobioreactors, but also a better understanding of the cyanobacterial physiology, coupled with the use of systems and synthetic biology approaches. Here, we briefly review recent advances in cyanobacterial biotechnology. We elaborate on some of the open problems in the field and call attention to the need for systems-level metabolic optimization endeavors in order to further develop these promising biocatalysts.
Cyanobacteria as cell-factories
Cyanobacteria are able to synthesize an array of value added compounds and they have been used as a source of drugs, toxins and fine chemicals for decades.4 The dawn of the genomic age, recent developments in genetic tools and the need to find alternatives to oil-based products have resulted in a significant increase in biotechnological studies of cyanobacteria in the last five years. These recent efforts have been targeted at the production of: (i) alcohols and related biofuel compounds, (ii) lipid based biofuels, (iii) sugars, (iv) biomaterials and (v) high value compounds (Table S1, ESI†).Alcohols and related biofuel compounds
Many cyanobacteria produce small quantities of ethanol; however, they lack the repertory of NADH-dependent fermentative pathways required to synthesize ethanol and other biofuel-like compounds at high titers. Compounding the problem is a NAD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NADP ratio, ranging from 1
NADP ratio, ranging from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to 1
10 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, depending on the growth conditions. The NAD(P)H/NAD(P) reduction states are likely to be important factors as well but more studies are needed since the results reported so far are conflicting.16–18 These properties have significant implications for biofuel production in cyanobacteria due to the preference for NADH of most of the biofuel biosynthetic pathways. In the last decade, several non-native pathways have been introduced in cyanobacteria, resulting in the successful production of a number of alcohol based biofuels. Deng and Coleman first demonstrated the feasibility of this engineered “photofermentative” metabolism. By introducing pyruvate decarboxylase and alcohol dehydrogenase II from Zymomonas mobilis in Synechococcus sp PCC 7942 (PCC7942), it was possible to produce up to 230 mg L−1 of ethanol.19 Although the yields were extremely low compared to heterotrophic organisms, significantly higher yields were obtained in later attempts20,21 (Table S1, ESI†). For instance, Gao et al. recently reported the production of up to 5500 mg L−1 of ethanol in Synechocystis sp PCC 6803 (PCC6803).21 The high productivity was achieved by overexpressing the pyruvate decarboxylase from Z. mobilis as well as the native NADPH-dependent alcohol dehydrogenase Slr1192, thereby overcoming the low NAD
5, depending on the growth conditions. The NAD(P)H/NAD(P) reduction states are likely to be important factors as well but more studies are needed since the results reported so far are conflicting.16–18 These properties have significant implications for biofuel production in cyanobacteria due to the preference for NADH of most of the biofuel biosynthetic pathways. In the last decade, several non-native pathways have been introduced in cyanobacteria, resulting in the successful production of a number of alcohol based biofuels. Deng and Coleman first demonstrated the feasibility of this engineered “photofermentative” metabolism. By introducing pyruvate decarboxylase and alcohol dehydrogenase II from Zymomonas mobilis in Synechococcus sp PCC 7942 (PCC7942), it was possible to produce up to 230 mg L−1 of ethanol.19 Although the yields were extremely low compared to heterotrophic organisms, significantly higher yields were obtained in later attempts20,21 (Table S1, ESI†). For instance, Gao et al. recently reported the production of up to 5500 mg L−1 of ethanol in Synechocystis sp PCC 6803 (PCC6803).21 The high productivity was achieved by overexpressing the pyruvate decarboxylase from Z. mobilis as well as the native NADPH-dependent alcohol dehydrogenase Slr1192, thereby overcoming the low NAD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NADP ratio. Atsumi and colleagues had previously employed a similar approach to produce isobutyraldehyde and isobutanol from pyruvate in PCC7942 by expressing a synthetic pathway including an acetoacetate synthase (AlsS), an acetohydroxy acid isomeroreductase (IlvC), a dihydroxy-acid dehydratase (IlvD), a ketoacid decarboxylase (Kivd) and an NADPH-dependent alcohol dehydrogenase (YqhD).22 They obtained increased yields of isobutyraldehyde by keeping the concentration low, reducing the toxicity to the cells, by in situ product removal. In a later study, the Liao group increased the yield of isobutanol by a factor of 2.5 by blocking the glycogen biosynthetic pathway which is a major sink of carbon in the autotrophic metabolism.23
NADP ratio. Atsumi and colleagues had previously employed a similar approach to produce isobutyraldehyde and isobutanol from pyruvate in PCC7942 by expressing a synthetic pathway including an acetoacetate synthase (AlsS), an acetohydroxy acid isomeroreductase (IlvC), a dihydroxy-acid dehydratase (IlvD), a ketoacid decarboxylase (Kivd) and an NADPH-dependent alcohol dehydrogenase (YqhD).22 They obtained increased yields of isobutyraldehyde by keeping the concentration low, reducing the toxicity to the cells, by in situ product removal. In a later study, the Liao group increased the yield of isobutanol by a factor of 2.5 by blocking the glycogen biosynthetic pathway which is a major sink of carbon in the autotrophic metabolism.23
Many non-native pathways exhibit high sensitivity to oxygen, reducing the possibility of heterologous expression in oxygenic photosynthetic organisms. Lan and Liao transferred a modified CoA-dependent 1-butanol pathway into PCC7942, obtaining 14.5 mg L−1 of 1-butanol in the dark under anoxic conditions.24 No 1-butanol production was observed under photosynthetic conditions which was attributed to a lack of a thermodynamic driving force and oxygen sensitivity of the enzymes in the pathway.24 In a follow-up study Lan and Liao engineered an ATP driving force by expressing an acetoacetyl-CoA synthase25 resulting in 6.5 mg L−1 of 1-butanol under photosynthetic conditions. By utilizing a NADPH-dependent acetoacetyl-CoA synthase and 3-ketobutyryl-CoA reductase in the pathway, the final titer reached 30 mg L−1.25 In their most recent work, Lan and Liao addressed the oxygen sensitivity of the pathway by replacing the CoA-acylating butyraldehyde dehydrogenase with a CoA-acylating propionaldehyde dehydrogenase, resulting in 400 mg L−1 of 1-butanol.26 By combining an oxygen-insensitive pathway, cofactor optimization and the introduction of irreversible enzymatic steps, 2.38 g L−1 of 2,3-butanediol were obtained from CO2.27 The above examples serve to illustrate the importance of taking the specific properties of the phototrophic metabolism into account in the metabolic engineering of cyanobacteria.
Lipid-based biofuels
Oleaginous algae have largely dominated the production of lipid based biofuels until now because of their ability to produce high amounts of triacylglycerol.28 However, cells containing triacylglycerol require complex and expensive downstream processing.3 Cyanobacteria have recently been engineered to produce and secrete free fatty acids and long chain alkenes into the culture medium and they may turn out to be a viable alternative to algae. Liu and colleagues employed six successive rounds of genetic modifications of PCC6803 and achieved 200 mg L−1 of extracellularly secreted fatty acids29 (Table S1, ESI†). The modifications included overproduction of acetyl-CoA carboxylase to funnel more carbon flux to fatty acids biosynthesis, the heterologous expression of engineered thioesterases, knockouts of genes encoding for competing pathways, e.g. cyanophycin biosynthesis, and weakening of the cell wall layer.Non-native fatty alcohol biosynthetic pathways, including heterologous fatty acyl-CoA reductases (FARs) from different sources, were used to produce hexadecanol, octadecanol and other fatty alcohols in PCC6803.30 The most promising producer strain included a FAR from jojoba, resulting in up to 10 μg OD−1 L−1 (≈26.2 μg gDW−1) of fatty alcohols and scale-up experiments revealed that the production of fatty alcohols was effectively doubled under high light conditions. A follow-up study by the same group reported significantly improved yields, 761 μg gDW−1. This was achieved by knocking out competing pathways (e.g., glycogen biosynthesis), promoter engineering and overexpressing multiple FARs.31
Long chain alka(e)nes are produced naturally by several cyanobacterial species as part of their lipidic membranes (up to 0.1% of the cell dry weight). They are ideal biofuels for several reasons. They have minimal downstream processing requirements, good combustion properties and the infrastructure for storage and distribution is already in place. An alkane biosynthesis pathway in cyanobacteria involving an acyl–acyl carrier protein reductase (AAR) and an aldehyde decarbonylase (AD) was recently discovered.32 The overexpression of these enzymes together with an acetyl-CoA carboxylase in PCC6803 resulted in 26 mg L−1 of alka(e)nes.33 Furthermore, the overexpression of a class-3 aldehyde-dehydrogenase in conjunction with AAR and AD in PCC794234 shifted the production from alkanes towards fatty acids, with the fatty acids being secreted from the cell. It has been shown that the production of fatty acids in cyanobacteria induces oxidative stress, which could limit both the efficiency and the lifetime of the biocatalyst. Comparative transcriptomics analysis identified up to 15 genes involved in fatty acid-induced stress defense in PCC7942.35 Interestingly, targeted mutagenesis and/or overexpression of some of the genes reduced fatty acid toxicity and subsequently led to an increase in fatty acid production.
Sugars
Cyanobacteria accumulate high levels of sucrose as an osmoprotectant under salt stress.36 This property, combined with the overexpression of key enzymes involved in sucrose biosynthesis, has been used in PCC6803 to obtain a significant increase in intracellular sucrose levels.37 Niederholtmeyer and colleagues obtained secretion of glucose and fructose in PCC7942 by overexpressing both invertase InvA, which produces glucose and fructose from sucrose, and the GLF sugar transport.38 Ducat et al. expressed a CscB-dependent sucrose export system from E. coli, knocked out invertase InvA and blocked the glycogen biosynthesis pathway and obtained up to 10 mM (approx. 3.5 g L−1) of sucrose under osmotic stress.39 The scope of sugar production continues to grow. For instance, a synthetic pathway for mannitol biosynthesis was recently expressed in Synechoccocuus sp. PCC 7002 (PCC7002), yielding up to 10% of cell dry weight.40 By blocking glycogen biosynthesis, the yield increased to 32%.The potential of cyanobacteria to secrete sugars has been suggested to be a feasible strategy to genetically engineer multispecies microbial cell factories, where the cyanobacteria provide oxygen and organic substrates and the heterotroph partner acts as an efficient biocatalyst.41 Although Niederholtmeyer et al. found that the secreted sugars supported E. coli growth in a co-culture with PCC7942,38 theoretical estimates indicate that the development of highly efficient multispecies biocatalysts will be very challenging.42,43
Biomaterials and chemical building blocks
Many cyanobacteria accumulate polyhydroxybutyrate at a high rate under nitrogen and/or phosphorus starvation.44 However, the addition of external carbon sources such as acetate was frequently required to achieve yields comparable to those found in heterotrophs. Recent efforts combining inverse metabolic engineering with high-throughput screening,45 as well as systems biology approaches, including transcriptomic and carbon flux rerouting, have been successfully applied, resulting in a significant increase in yields.46 In addition, the production and extracellular secretion of the polyhydroxybutyrate intermediate (S),(R)-3-hydroxybutyrate has been achieved in PCC6803 under autotrophic conditions.47Isoprene is a versatile building block derived from crude oil, which is mainly used for synthetic rubber but also in flavorings and perfumes. Small amounts of isoprene in PCC6803 were obtained by the heterologous expression of a codon optimized version of the ispS gene from kudzu, encoding for the isoprene synthase.48 Ethylene, another major building block in the chemical industry has received considerable interest. For instance, ethylene has been produced in PCC7942 harboring the Ethylene-Forming Enzyme (EFE) from Pseudomonas syringae.49 However, the production of ethylene was not stable over time, a frequent problem in the metabolic engineering of cyanobacteria. This was due to recurrent mutations of the encoding gene, even under inducible expression.50 In two recent studies, stable and continuous production of ethylene was achieved through rigorous codon use and promoter optimization.51,52 While these results are promising, the best yields achieved so far are 171 mg L−1 per day52 and further efforts are clearly needed.
L-Lactate has been produced in titers up to 290 mg L−1 in PCC6803, by expressing the ldh gene from Bacillus subtilis and a soluble transhydrogenase.53 In a later study Angermayr and Hellingwerf used metabolic control analysis to show that L-lactic production was linearly dependent on the lactate dehydrogenase enzymatic capacity. Significantly higher yields were achieved by expressing the ldh gene of Lactococcus lactis under the control of the promoter trc54 (Table S1, ESI†). Finally, D-lactate has been obtained by expressing a mutated glycerol dehydrogenase with D-lactate dehydrogenase activity in PCC6803. A titer of 1140 mg L−1 was achieved by increasing the NADH pool through the expression of a soluble transhydrogenase and codon optimization.55
High value compounds
Given the low yields of metabolites obtained in cyanobacteria so far, a reasonable alternative is to focus biotechnological efforts on the production of high-value compounds instead of high-volume, low-value compounds such as biofuels.43 In a pioneering study, Yu et al., obtained 2.24 mg L−1 of eicosapentaenoic acid (EPA), a polyunsaturated fatty acid of clinical importance, by expressing the EPA biosynthetic pathway from Shewanella sp. SCRC2738 in Synechococus sp. NKBG15041c.56 Reinsvold and colleagues engineered a recombinant PCC6803 strain harboring the β-caryophyllene synthase gene (QHS1) from Artemisia annua resulting in the production of the non-native secondary metabolite β-caryophyllene which is used in the cosmetic industry.57 Squalene, a 30-carbon natural isoprenoid, used in cosmetics and vaccines, was successfully produced in PCC6803 by disrupting the hopanoid biosynthetic pathway.58Metabolic features specific to cyanobacterial networks
It is evident from the above discussion that there is a growing interest in the use of cyanobacteria as biocatalysts. Many foundational problems have been addressed (Box 1), resulting in an increased variety of target chemicals, as well as in improved titers (Table S1, ESI†). However, there is a long way to go before cyanobacteria can be widely applied as biocatalysts in industrial settings. Concerns stem from the many problems that are still unsolved, including low yields and the difficulties in designing efficient photobioreactors.43,59 It is not our aim here to discuss in detail all of the challenges facing cyanobacterial biotechnology since excellent reviews have already been published.43,60–63 We focus instead on the metabolic features specific to cyanobacterial networks and how they affect the potential use of cyanobacteria as efficient biocatalysts.
Box 1. Foundational problems in cyanobacterial biotechnology, the approaches that are currently used to address them and potential approaches based on systems biology
| ||||||||||||||||||||||||||||||||||||||||||||||||
Knowledge gaps
Although cyanobacteria have been studied in considerable detail, PCC6803 in particular, knowledge about their metabolism is lacking in many aspects, even the central metabolism. For instance, the TCA cycle was considered “incomplete” for almost 50 years because alpha-ketoglutarate dehydrogenase (AKGDH) is missing, and only very recently has an alternative pathway been identified.64 For the most widely used organisms in biotechnology, such as E. coli, there exist a large number of knowledge bases, including metabolic,65 gene-expression66 and transcriptional regulation67 databases, together with extensive gene knock-out68 and gene knock-in69 libraries. In comparison, the availability of cyanobacterial resources is fairly limited70,71 and cyanobacteria-specific genetic tools have only recently become available.72,73 From successful engineering projects involving E. coli and yeast it is clear that improving productivity requires deep physiological, genetic and metabolic characterization of the host strain, as well as strain-specific genetic tools.60,74 Therefore, it is reasonable to assume that the metabolic engineering efforts undertaken in cyanobacteria until now have been hampered by significant knowledge gaps. This may explain to some extent the relatively small number of target chemicals explored so far and the low titers obtained in most of the studies.Chemical space
An analysis of the chemical space covered by metabolically engineered cyanobacteria illustrates that many targets of industrial importance, such as dicarboxylic acids, organic acids and amino acids in particular, remain to be explored (Table S1, ESI,† and Fig. 1a). While a significant portion of the chemical space relevant to industry has already been covered in metabolic engineering workhorses such as E. coli, the space covered by cyanobacteria is mostly restricted to alcohols, a few organic acids, sugars and terpenes (Fig. 1a). It is likely that the limited coverage is mainly due to a lack of attempts so far and the recent example of p-coumaric acid production from tyrosine in PCC680375 demonstrates how the scope can be extended to a new family of chemicals such as aromatic acids.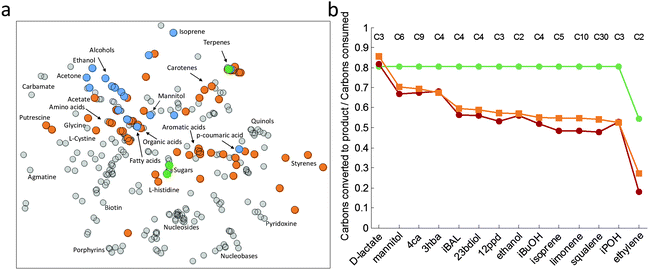 | ||
| Fig. 1 Maximum theoretical yields and the chemical space covered by cyanobacteria. (a) The chemical space covered by cyanobacteria and E. coli. The figure represents chemical similarities between compounds in such a way that two points that are close together have a similar chemical structure while compounds that are separated by a large distance are dissimilar. The cyanobacterial metabolites (gray points) and metabolic engineering targets in cyanobacteria (blue and green points) and E. coli (blue and orange points) reveal that the productive potential of cyanobacteria is largely unexplored compared to E. coli. The cyanobacteria targets fall mostly into the top left quadrant whereas the E. coli targets cover a considerably larger area. The axis units are arbitrary. Figure details: the metabolites for the cyanobacteria were derived from metabolic reconstructions of Synechocystis sp. PCC6803,12Cyanothece sp. ATCC 5114213 and Spirulina platensis C114 after removing unstable intermediates. They correspond to carbon containing compounds with KEGG IDs and matching IUPAC International Chemical Identifiers. Chemical similarities between the compounds are represented by Tanimoto coefficients derived from FP2 path-based fingerprints of the compounds obtained using the obabel program.116 The resulting 364 by 364 similarity matrix was visualized using the t-SNE algorithm.117 (b) Maximum theoretical yields of selected compounds in Synechocystis under autotrophic conditions (green), heterotrophic conditions (red) and E. coli growing on glucose (orange). The yields are defined as the ratio of the number of carbon atoms converted to the target product versus the number of carbon atoms consumed. The low yields of ethylene are due to the generation of guanidine, a byproduct that does not appear to be metabolized. Figure details: the reconstructed networks of Synechocystis, iJN678,12 and E. coli, iJO1366,118 were used to calculate the theoretical yields after adding the necessary pathways to the models and fixing the biomass to 20% of the maximum. Light uptake under autotrophic conditions corresponded to the amount required for maximal growth in order to avoid unrealistic energy production due to extra light uptake. Abbreviations: 1,2-propanediol (12ppd), p-coumaric acid (4ca), (R)-3-hydroxybutyrate (3hba), isobutanol (iBuOH), and isobutyraldehyde (iBAL). | ||
Theoretical yields
The low yields frequently reported in metabolic engineering studies involving cyanobacteria raise the question of whether they are caused by some inherent limitations of the metabolic network. To investigate this possibility, we computed the theoretical yields for selected chemicals. A genome-scale model of PCC6803, iJN678, was used to compute the maximum yields under auto- and heterotrophic conditions and the yields were then compared to E. coli. The yields were defined as the number of carbon atoms converted to the target product versus the number of carbon atoms consumed. The metabolic burden of the biosynthetic pathways was taken into account by requiring a certain amount of biomass to be produced at the same time as previously reported.99 Accordingly, here the fraction was set to 20% of the maximum. Qualitatively similar results were obtained with other values of the biomass fraction. Under heterotrophic conditions the yields resembled those of E. coli, ranging from 0.53 (ethylene) to 0.86 (lactate). With the exception of lactate, the loss of CO2 results in a significant decrease in yields in E. coli. Interestingly, the yields were very stable under autotrophic conditions and considerably higher in PCC6803 than in E. coli (Fig. 1b). The reason is that the light-driven metabolism avoids the loss of carbon in the form of CO2. This is an exclusive trait of CO2 fixing organisms76 and it represents an important advantage over heterotrophs. The conservation of carbon increases the productivity of the target compound since carbon fixation is a major bottleneck in biotechnology.63 The analysis also showed that chemical production required fewer photons than biomass production, a finding previously reported by Maarleveld et al.77 This indicates that increased product yields will lead to better light usage which can explain, to some extent, the high CO2 fixation ratios found in several overproducer strains.39 In summary, the high theoretical yields obtained under autotrophic conditions suggest that the topology of the photosynthetic metabolic networks, per se, is not directly responsible for the low yields obtained so far in cyanobacteria.Carbon flux
An important but often overlooked issue in metabolic engineering efforts is the unique nature of cyanobacterial metabolic networks. For instance, the carbon flux and carbon partitioning in the central metabolism under autotrophic conditions have only recently been determined.78 It was shown that under autotrophic conditions, the total CO2 fixed in the form of 3-phosphoglycerate (3PG) is split between phosphoglycerate mutase and phosphoglycerate kinase in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. A similar ratio was later predicted using a computational approach.12 This carbon flux distribution differs considerably from heterotrophic metabolism and as a consequence only one out of ten CO2 molecules that are fixed is funneled to pyruvate. In addition, studies carried out by the Melis group suggest that up to 80% of the carbon is funneled to sugar biosynthesis while only 5% and 10% are allocated to biotechnologically relevant pathways such as terpenoid and fatty acid biosynthesis, respectively (unpublished results).48,79 The flux distribution present in cyanobacteria appears to be a consequence of the high carbon flux across the Calvin cycle which is required to optimize CO2 fixation. Furthermore, since respiration is not needed for energy production under autotrophic conditions, the carbon flux towards important biotechnological precursor metabolites, such as pyruvate and tricarboxylic acids, is reduced.43 This intrinsic difference with respect to heterotrophic networks is likely to be one of the reasons why attempts to transfer existing engineering strategies from heterotrophic organisms to cyanobacteria have had limited success so far. It has been suggested that in order to maximize the carbon partitioning towards the target product, a heterologous pathway should obtain the carbon as close as possible to the fixation pathway.61 Interestingly, genetic engineering approaches which have focused on increasing the driving-force of the synthetic pathway have increased the carbon flux to the key precursor, pyruvate (which is only three steps away from 3PG), resulting in significant yields for ethanol (≈70%)21 and 2,3-butanediol (>60%).27 Although the issue of which precursors are best to “tap” from is unresolved, the above discussion indicates that achieving comparable productivity for other industrially relevant chemicals whose synthesis starts far away from 3PG may be a considerable challenge.
10. A similar ratio was later predicted using a computational approach.12 This carbon flux distribution differs considerably from heterotrophic metabolism and as a consequence only one out of ten CO2 molecules that are fixed is funneled to pyruvate. In addition, studies carried out by the Melis group suggest that up to 80% of the carbon is funneled to sugar biosynthesis while only 5% and 10% are allocated to biotechnologically relevant pathways such as terpenoid and fatty acid biosynthesis, respectively (unpublished results).48,79 The flux distribution present in cyanobacteria appears to be a consequence of the high carbon flux across the Calvin cycle which is required to optimize CO2 fixation. Furthermore, since respiration is not needed for energy production under autotrophic conditions, the carbon flux towards important biotechnological precursor metabolites, such as pyruvate and tricarboxylic acids, is reduced.43 This intrinsic difference with respect to heterotrophic networks is likely to be one of the reasons why attempts to transfer existing engineering strategies from heterotrophic organisms to cyanobacteria have had limited success so far. It has been suggested that in order to maximize the carbon partitioning towards the target product, a heterologous pathway should obtain the carbon as close as possible to the fixation pathway.61 Interestingly, genetic engineering approaches which have focused on increasing the driving-force of the synthetic pathway have increased the carbon flux to the key precursor, pyruvate (which is only three steps away from 3PG), resulting in significant yields for ethanol (≈70%)21 and 2,3-butanediol (>60%).27 Although the issue of which precursors are best to “tap” from is unresolved, the above discussion indicates that achieving comparable productivity for other industrially relevant chemicals whose synthesis starts far away from 3PG may be a considerable challenge.
Light metabolism
A unique property of the photoautotrophic metabolism is its dependence on light for energy supply. In addition to the photosynthetic linear electron flow pathway, phototrophs are equipped with a large number of alternate electron flow pathways which assist in balancing the ATP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NADPH ratio as a function of metabolic demand.12,80 This flexibility, or robustness of the photosynthetic system, allows for fine-tuning of light to energy conversion by photosystems I and II and the metabolic reactions and provides an ATP
NADPH ratio as a function of metabolic demand.12,80 This flexibility, or robustness of the photosynthetic system, allows for fine-tuning of light to energy conversion by photosystems I and II and the metabolic reactions and provides an ATP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NADPH ratio close to 1.5 which is required for optimal carbon fixation. In addition, systems biology studies employing gene essentiality prediction have suggested that cyanobacteria possess significantly reduced metabolic robustness compared to heterotrophic organisms12,81 and that there is a trade-off between high photosynthetic robustness and low metabolic robustness.12 If these predictions are confirmed, the above properties would have a significant impact on biotechnology. First, the existence of multiple electron flow pathways involved in ATP and redox balancing limits the effects of the extreme ATP
NADPH ratio close to 1.5 which is required for optimal carbon fixation. In addition, systems biology studies employing gene essentiality prediction have suggested that cyanobacteria possess significantly reduced metabolic robustness compared to heterotrophic organisms12,81 and that there is a trade-off between high photosynthetic robustness and low metabolic robustness.12 If these predictions are confirmed, the above properties would have a significant impact on biotechnology. First, the existence of multiple electron flow pathways involved in ATP and redox balancing limits the effects of the extreme ATP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NADPH ratios required to produce non-native chemicals.61 However it is important to keep in mind that the photosynthetic robustness could also limit the use of biotechnologically relevant pathways as non-native electron sinks, a strategy that otherwise could be used to increase yields. Second, although reduced metabolic robustness could be an advantage for carbon flux rerouting since it implies fewer redundant and/or competing pathways, it also limits the possibilities of removing non-desirable, potentially toxic byproducts from engineered pathways. The byproducts may contribute to the genetic instability found often in many of the heterologous pathways explored in cyanobacteria. The systems properties described above have so far mostly been unexplored in metabolic engineering efforts. Systems biology opens up the possibility of engineering these properties which could in turn increase the applicability of cyanobacteria as cell-factories.
NADPH ratios required to produce non-native chemicals.61 However it is important to keep in mind that the photosynthetic robustness could also limit the use of biotechnologically relevant pathways as non-native electron sinks, a strategy that otherwise could be used to increase yields. Second, although reduced metabolic robustness could be an advantage for carbon flux rerouting since it implies fewer redundant and/or competing pathways, it also limits the possibilities of removing non-desirable, potentially toxic byproducts from engineered pathways. The byproducts may contribute to the genetic instability found often in many of the heterologous pathways explored in cyanobacteria. The systems properties described above have so far mostly been unexplored in metabolic engineering efforts. Systems biology opens up the possibility of engineering these properties which could in turn increase the applicability of cyanobacteria as cell-factories.
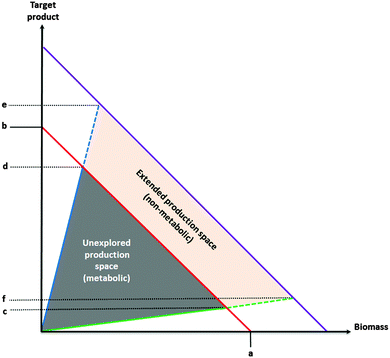
In summary, it seems reasonable to assume that in addition to well-known optimization targets, such as photosynthetic efficiency, carbon fixation and bioreactor design, deeper knowledge of the metabolism and physiology at the systems level will lead to further advances in cyanobacterial biotechnology. Systems metabolic engineering approaches have successfully been applied to heterotrophic hosts to modify carbon partitioning and to increase yields.74,82 Such approaches have yet to be applied to cyanobacteria, suggesting that a large part of the metabolic “production space” is yet to be explored (Fig. 2). Systems approaches are likely to play an important role in future efforts, since the resulting gains are mostly independent of improvements of the classical optimization targets (Fig. 2).
Taking advantage of systems biology in cyanobacterial biotechnology
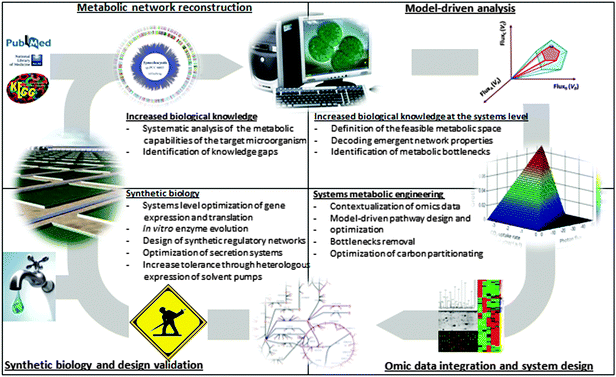
The metabolic optimization of cyanobacteria as biocatalysts requires a systems level understanding of their metabolic and physiological processes as well as cyanobacterial-specific metabolic engineering designs. Genome-scale metabolic network reconstructions (Box 2) may turn out to be essential in achieving these goals. These reconstructions have been extremely useful as platforms for biological knowledge discovery and contextualization of omics data,83 and they are increasingly being used in metabolic engineering.84 Metabolic reconstructions now exist for several cyanobacterial species, PCC6803,12,81,85–89 PCC7002,90Cyanothece sp. ATCC51142 (ATCC51142)13,88 and Spirulina platensis C1.14 The earliest models involved mainly the central metabolism, but later reconstructions included detailed modeling of photosynthetic processes, the diurnal cycle, synthesis of lipids and photosynthetic pigments.10,12,13 In addition, 35 cyanobacterial metabolic networks have been reconstructed from their respective genome sequences using an automatic algorithm.91 It is reasonable to expect that the arrival of cyanobacteria reconstructions and the emergence of synthetic biology will play key roles in resolving the multiple problems that currently hamper the biotechnological potential of cyanobacteria. Until now, the cyanobacteria models have been used for three main proposes: (i) as tools for increasing biological knowledge, (ii) as platforms for omics data integration and contextualization and (iii) as a test bed for biotechnological applications.
Box 2. Genome-scale metabolic reconstructionsSystems biology attempts to obtain a detailed understanding of biological processes by a bottom-up approach where biochemical information about sub-cellular processes is integrated to form computational models of cellular activity at higher levels, culminating in comprehensive models of single cell activity or even groups of cells. The computational nature of the models enables quantitative predictions of cellular behavior under different conditions. Models of metabolism in many prokaryotic and eukaryotic organisms have been constructed112 and models of transcription, regulation and signaling networks have also been developed, although to a lesser extent.113 Multi-scale models combining different types of networks, e.g. metabolic and transcription/translation networks,114 are starting to become available and are expected to increase the predictive accuracy even further.Genome-scale metabolic models are constructed from genetic, genomic and biochemical data obtained from online databases and primary literature, following a standardized protocol.115 The models enable quantitative predictions in terms of fluxes through individual reactions. The predictions are frequently made using flux balance analysis, a computational algorithm which calculates flux values in all the reactions corresponding to a particular cellular objective such as the maximization of biomass, production of ATP or synthesis of the target compound.99 The effects of heterologous gene insertion are easily simulated by adding the corresponding reaction(s) to the model. An example is the production of lactate, a non-native compound in Synechocystis. In this case, reactions describing the synthesis of lactate from pyruvate (via lactate dehydrogenase) and the transport of lactate out of the cell would be added. A gene knockout is simulated by simply removing the corresponding reaction(s) from the model. |
Models as tools for increasing biological knowledge
Metabolic reconstructions are increasingly being used to gain insights into cyanobacterial metabolism and the TCA cycle has been studied in some detail. Nogales et al. proposed the GABA shunt as an alternative to close the TCA cycle in PCC6803 and computational analysis suggested that the GABA shunt provided an advantage over AKGDH under photoautotrophic conditions.12 Knoop et al. evaluated several alternatives proposed to close the TCA cycle, and provided computational and experimental evidence for the absence of a functional glyoxlyate shunt.10 These computational studies have recently been validated and the important role of the GABA shunt in closing the TCA cycle in PCC6803 has been demonstrated, despite the presence of the alternative TCA shortcut as well.64,92,93 By studying the photosynthetic processes in PCC6803 under varying light and carbon conditions and analyzing network robustness under genetic perturbations, it was concluded that high photosynthetic robustness, including multiple alternate electron flow pathways, is required for optimal photosynthetic performance and that this comes at the expense of reduced metabolic robustness.12 Building on these initial systems-level analyses of photosynthetic networks, future studies employing metabolic models are likely to increase our current understanding of cyanobacterial metabolism and facilitate metabolic engineering efforts (Fig. 3).Models as platforms for omics data integration and contextualization
In recent years the biological sciences have been hit by a data avalanche in the form of “omics” data sets. Transcriptomics, proteomics, fluxomics and metabolomics data are now routinely collected during biological experiments. Metabolic reconstructions provide a very useful context for omics data sets since they enable mechanistic interpretation of the data.83,94 The resulting condition-specific models can be used to prioritize hypothesis for experimental validation, may lend support to observations that are otherwise difficult to validate experimentally, and lead to biological discovery and more accurate metabolic engineering designs.83Several condition-specific models already exist for cyanobacteria, and they have been used to increase our understanding of cyanobacterial physiology.10,13,88,90 Protein expression data over light and dark phases were used to model the diurnal rhythm of ATCC51142 by Saha et al.88 Vu et al. applied mRNA and protein expression datasets to construct light and ammonium limited models of ATCC51142. The condition-specific models not only reduced the prediction uncertainty, but they also guided the discovery of proline as an alternative nitrogen source.13 By combining metabolic and transcriptomic networks, Montagud et al. identified the first steps of pyrimidine synthesis and oxidative phosphorylation as regulatory hubs for transcriptional changes in light availability in PCC6803.95 Knoop et al. modeled the diurnal cycle using measurements of transcriptional expression in response to light variability. This approach allowed the dynamic simulation and estimation of carbon flux in PCC6803 as a function of light availability during the day.10 The potential of these condition specific models in biotechnology is largely untapped. A large number of transcriptome data sets collected for PCC6803 have recently been compiled96 and await further study in the context of metabolic networks. The same holds true for a recently published web-based database for interactive exploration and visualization of transcriptomic data in PCC6803.70
Models as a test bed in biotechnological applications
Several algorithms for metabolic engineering based on network reconstructions are available, including algorithms that couple the secretion of the target compound to growth by gene knockouts.97,98 Growth-coupling is a highly desirable trait since it alleviates the problems of selection and genetic instability and enables the use of adaptive evolution to further increase the production rate.99 Algorithms such as OptForce100 can be used to identify which fluxes must increase or decrease to achieve a pre-specified overproduction target. In addition, algorithms for designing synthetic pathways can be used to optimize the production of native and non-native compounds and to devise strategies for the removal of toxic byproducts.101 Such algorithms have been instrumental in the success of several recent metabolic engineering projects including the overproduction of 1,4-butanediol74 and L-valine82 in E. coli.In silico studies of the capabilities of cyanobacteria for chemical production have mostly focused on the overproduction of hydrogen81,88 and ethanol.86–88 Other computational studies have demonstrated some of the significant challenges involved in the use of cyanobacteria as biocatalysts and have identified key bottlenecks. A search for growth-coupled knockout strains in PCC6803 showed that carbon flux re-routing was considerably more difficult under autotrophic conditions than under mixotrophic and heterotrophic conditions.102 Furthermore, for growth-coupling under mixotrophic conditions it was necessary to reduce the photosynthetic robustness by blocking the light-driven metabolism but in this case net CO2 fixation was absent for most of the strains and the mixotrophic metabolism resembled that of heterotrophs.102 Vu et al. studied the capabilities of PCC7002 for producing several native and non-native compounds, including succinate, alanine, isoprene, butanol and ethanol.90 Computational experiments showed that single deletions in the central metabolism were predicted to improve the production of the target chemicals but the production was not coupled to growth. The computational search for growth coupled mutants found that a large number of knockouts were needed under autotrophic conditions in both the strains; most strategies required 9 to 10 deletions.12,90 High-quality models of cyanobacteria have only recently become available103 and their uses in metabolic engineering appear to be limited to computational analysis. To the best of our knowledge, no model-driven experimental attempts to overproduce chemicals have been undertaken so far; however, the implementation of these computational approaches is now possible.
Outlook
Significant advances have been made in cyanobacterial biotechnology in the last few years. The field has matured rapidly and it is now possible to use cyanobacteria for sustainable production of biofuels and fine chemicals. However, the current approaches are still far from being economically feasible and are unlikely to replace crude oil-based processes in the near future. Many challenges remain and multiple steps need to be optimized before phototroph-based biotechnology becomes competitive, such as photosynthetic efficiency and photobioreactor design.43,59,104 The optimization of the cyanobacterial metabolism has barely begun. Increasing our knowledge about the metabolism in cyanobacteria is likely to lead to significant improvements in strain design in the same way as happened with E. coli and yeast. Existing metabolic reconstructions are extremely useful tools for these purposes. To obtain further insights into biotechnologically relevant processes, the next generation of models needs to include additional modules, such as reactive oxygen species, and a more comprehensive diurnal cycle description. The effects of varying light wavelengths can be modeled in a fairly straightforward manner105 and light-quality could then be included as an additional environmental factor in future model-driven biotechnology efforts. These systems biology efforts, combined with modern synthetic biology approaches, may lead to the long awaited economic feasibility of cyanobacterial cell factories (Box 2).Acknowledgements
This work was supported by the SYNPOL (FP7-KBBE 311815; http://www.synpol.org/) EU project. The authors would like to thank the anonymous reviewers for their critical comments and valuable suggestions, which improved the quality of the paper considerably.References
- J. M. Melillo, et al. , Science, 2009, 326, 1397–1399 CrossRef CAS PubMed.
- M. A. Delucchi, Ann. N. Y. Acad. Sci., 2010, 1195, 28–45 CrossRef CAS PubMed.
- E. Molina Grima, E. H. Belarbi, F. G. Acién Fernández, A. Robles Medina and Y. Chisti, Biotechnol. Adv., 2003, 20, 491–515 CrossRef CAS PubMed.
- N. W. Lem and B. R. Glick, Biotechnol. Adv., 1985, 3, 195–208 CrossRef CAS PubMed.
- R. M. M. Abed, S. Dobretsov and K. Sudesh, J. Appl. Microbiol., 2009, 106, 1–12 CrossRef CAS PubMed.
- R. Y. Stanier and G. C. Bazine, Annu. Rev. Microbiol., 1977, 31, 225–274 CrossRef CAS PubMed.
- N. Quintana, F. Kooy, M. Rhee, G. Voshol and R. Verpoorte, Appl. Microbiol. Biotechnol., 2011, 91, 471–490 CrossRef CAS PubMed.
- G. C. Dismukes, D. Carrieri, N. Bennette, G. M. Ananyev and M. C. Posewitz, Curr. Opin. Biotechnol., 2008, 19, 235–240 CrossRef CAS PubMed.
- X.-G. Zhu, S. P. Long and D. R. Ort, Curr. Opin. Biotechnol., 2008, 19, 153–159 CrossRef CAS PubMed.
- H. Knoop, et al. , PLoS Comput. Biol., 2013, 9, e1003081 CAS.
- T. Mueller, B. Berla, H. Pakrasi and C. Maranas, BMC Syst. Biol., 2013, 7, 142 CrossRef PubMed.
- J. Nogales, S. Gudmundsson, E. M. Knight, B. O. Palsson and I. Thiele, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 2678–2683 CrossRef CAS PubMed.
- T. T. Vu, et al. , PLoS Comput. Biol., 2012, 8, e1002460 CAS.
- A. Klanchui, C. Khannapho, A. Phodee, S. Cheevadhanarak and A. Meechai, BMC Syst. Biol., 2012, 6, 71 CrossRef CAS PubMed.
- T. Heidorn, et al., in Methods Enzymol, ed. V. Chris, Academic Press, 2011, vol. 497, pp. 539–579 Search PubMed.
- M. Tamoi, T. Miyazaki, T. Fukamizo and S. Shigeoka, Plant J., 2005, 42, 504–513 CrossRef CAS PubMed.
- H. Takahashi, H. Uchimiya and Y. Hihara, J. Exp. Bot., 2008, 59, 3009–3018 CrossRef CAS PubMed.
- J. W. Cooley and W. F. J. Vermaas, J. Bacteriol., 2001, 183, 4251–4258 CrossRef CAS PubMed.
- M.-D. Deng and J. R. Coleman, Appl. Environ. Microbiol., 1999, 65, 523–528 CAS.
- J. Dexter and P. Fu, Energy Environ. Sci., 2009, 2, 857–864 CAS.
- Z. Gao, H. Zhao, Z. Li, X. Tan and X. Lu, Energy Environ. Sci., 2012, 5, 9857–9865 CAS.
- S. Atsumi, W. Higashide and J. C. Liao, Nat. Biotechnol., 2009, 27, 1177–1180 CrossRef CAS PubMed.
- X. Li, C. Shen and J. Liao, Photosynth. Res., 2014, 1–10 Search PubMed.
- E. I. Lan and J. C. Liao, Metab. Eng., 2011, 13, 353–363 CrossRef CAS PubMed.
- E. I. Lan and J. C. Liao, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 6018–6023 CrossRef CAS PubMed.
- E. I. Lan, S. Y. Ro and J. C. Liao, Energy Environ. Sci., 2013, 6, 2672–2681 CAS.
- J. W. K. Oliver, I. M. P. Machado, H. Yoneda and S. Atsumi, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 1249–1254 CrossRef CAS PubMed.
- Q. Hu, et al. , Plant J., 2008, 54, 621–639 CrossRef CAS PubMed.
- X. Liu, J. Sheng and R. Curtiss III, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 6899–6904 CrossRef CAS PubMed.
- X. Tan, et al. , Metab. Eng., 2011, 13, 169–176 CrossRef CAS PubMed.
- F. Qi, L. Yao, X. Tan and X. Lu, Biotechnol. Lett., 2013, 35, 1655–1661 CrossRef CAS PubMed.
- A. Schirmer, M. A. Rude, X. Li, E. Popova and S. B. del Cardayre, Science, 2010, 329, 559–562 CrossRef CAS PubMed.
- W. Wang, X. Liu and X. Lu, Biotechnol. Biofuels, 2013, 6, 69 CrossRef CAS PubMed.
- B. K. Kaiser, et al. , PLoS One, 2013, 8, e58307 CAS.
- A. Ruffing, Biotechnol. Biofuels, 2013, 6, 113 CrossRef CAS PubMed.
- M. Hagemann and K. Marin, J. Plant Physiol., 1999, 155, 424–430 CrossRef CAS.
- W. Du, F. Liang, Y. Duan, X. Tan and X. Lu, Metab. Eng., 2013, 19, 17–25 CrossRef CAS PubMed.
- H. Niederholtmeyer, B. T. Wolfstädter, D. F. Savage, P. A. Silver and J. C. Way, Appl. Environ. Microbiol., 2010, 76, 3462–3466 CrossRef CAS PubMed.
- D. C. Ducat, J. A. Avelar-Rivas, J. C. Way and P. A. Silver, Appl. Environ. Microbiol., 2012, 78, 2660–2668 CrossRef CAS PubMed.
- J. H. Jacobsen and N.-U. Frigaard, Metab. Eng., 2014, 21, 60–70 CrossRef CAS PubMed.
- J. C. F. Ortiz-Marquez, M. Do Nascimento, J. P. Zehr and L. Curatti, Trends Biotechnol., 2013, 31, 521–529 CrossRef CAS PubMed.
- C. M. Agapakis, et al. , PLoS One, 2011, 6, e18877 CAS.
- D. C. Ducat, J. C. Way and P. A. Silver, Trends Biotechnol., 2011, 29, 95–103 CrossRef CAS PubMed.
- Y. Asada, M. Miyake, J. Miyake, R. Kurane and Y. Tokiwa, Int. J. Biol. Macromol., 1999, 25, 37–42 CrossRef CAS PubMed.
- K. E. J. Tyo, Y.-S. Jin, F. A. Espinoza and G. Stephanopoulos, Biotechnol. Prog., 2009, 25, 1236–1243 CrossRef CAS PubMed.
- T. Osanai, et al. , DNA Res., 2013, 20, 525–535 CrossRef CAS PubMed.
- B. Wang, S. Pugh, D. R. Nielsen, W. Zhang and D. R. Meldrum, Metab. Eng., 2013, 16, 68–77 CrossRef CAS PubMed.
- P. Lindberg, S. Park and A. Melis, Metab. Eng., 2010, 12, 70–79 CrossRef CAS PubMed.
- M. Sakai, T. Ogawa, M. Matsuoka and H. Fukuda, J. Ferment. Bioeng., 1997, 84, 434–443 CrossRef CAS.
- K. Takahama, M. Matsuoka, K. Nagahama and T. Ogawa, J. Biosci. Bioeng., 2003, 95, 302–305 CrossRef CAS PubMed.
- F. Guerrero, V. Carbonell, M. Cossu, D. Correddu and P. R. Jones, PLoS One, 2012, 7, e50470 CAS.
- J. Ungerer, L. Tao, M. Davis, M. Ghirardi, P.-C. Maness and J. Yu, Energy Environ. Sci., 2012, 5, 8998–9006 CAS.
- S. A. Angermayr, M. Paszota and K. J. Hellingwerf, Appl. Environ. Microbiol., 2012, 78, 7098–7106 CrossRef CAS PubMed.
- S. A. Angermayr and K. J. Hellingwerf, J. Phys. Chem. B, 2013, 117, 11169–11175 CrossRef CAS PubMed.
- A. Varman, Y. Yu, L. You and Y. Tang, Microb. Cell Fact., 2013, 12, 1–8 CrossRef PubMed.
- R. Yu, et al. , Lipids, 2000, 35, 1061–1064 CrossRef CAS PubMed.
- R. E. Reinsvold, R. E. Jinkerson, R. Radakovits, M. C. Posewitz and C. Basu, J. Plant Physiol., 2011, 168, 848–852 CrossRef CAS PubMed.
- E. Englund, et al. , PLoS One, 2014, 9, e90270 Search PubMed.
- V. H. Work, S. D'Adamo, R. Radakovits, R. E. Jinkerson and M. C. Posewitz, Curr. Opin. Biotechnol., 2012, 23, 290–297 CrossRef CAS PubMed.
- N. E. Nozzi, J. W. K. Oliver and S. Atsumi, Front. Bioeng. Biotechnol., 2013, 1, 7, DOI:10.3389/fbioe.2013.00007.
- J. K. Oliver and S. Atsumi, Photosynth. Res., 2014, 120, 249–261 CrossRef CAS PubMed.
- K. J. Hellingwerf and M. J. Teixeira de Mattos, J. Biotechnol., 2009, 142, 87–90 CrossRef CAS PubMed.
- L. Rosgaard, A. J. de Porcellinis, J. H. Jacobsen, N.-U. Frigaard and Y. Sakuragi, J. Biotechnol., 2012, 162, 134–147 CrossRef CAS PubMed.
- S. Zhang and D. A. Bryant, Science, 2011, 334, 1551–1553 CrossRef CAS PubMed.
- I. M. Keseler, et al. , Nucleic Acids Res., 2013, 41, D605–D612 CrossRef CAS PubMed.
- E. coli Gene Expression Database (GenExpDB). <http://genexpdb.ou.edu/main/>.
- H. Salgado, et al. , Nucleic Acids Res., 2013, 41, D203–D213 CrossRef CAS PubMed.
- T. Baba, et al. , Mol. Syst. Biol., 2006, 2, 2006.0008 CrossRef PubMed.
- M. Kitagawa, et al. , DNA Res., 2006, 12, 291–299 CrossRef PubMed.
- M. A. Hernandez-Prieto and M. E. Futschik, Bioinformation, 2012, 8, 634–638 CrossRef PubMed.
- M. A. Hernández-Prieto, T. A. Semeniuk and M. E. Futschik, Front. Genet., 2014, 5, 191, DOI:10.3389/fgene.2014.00191.
- A. Taton, et al. , Nucleic Acids Res., 2015, 42, e136, DOI:10.1093/nar/gku673.
- B. M. Berla, R. Saha, C. M. Immethun, C. D. Maranas, T. S. Moon and H. Pakrasi, Front. Microbiol., 2013, 4, 246 Search PubMed.
- H. Yim, et al. , Nat. Chem. Biol., 2011, 7, 445–452 CrossRef CAS PubMed.
- Y. Xue, Y. Zhang, D. Cheng, S. Daddy and Q. He, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 9449–9454 CrossRef CAS PubMed.
- I. W. Bogorad, T.-S. Lin and J. C. Liao, Nature, 2013, 502, 693–697 CrossRef CAS PubMed.
- T. R. Maarleveld, J. Boele, F. J. Bruggeman and B. Teusink, Plant Physiol., 2014, 164, 1111–1121 CrossRef CAS PubMed.
- J. D. Young, A. A. Shastri, G. Stephanopoulos and J. A. Morgan, Metab. Eng., 2011, 13, 656–665 CrossRef CAS PubMed.
- A. Melis, Curr. Opin. Chem. Biol., 2013, 17, 453–456 CrossRef CAS PubMed.
- D. M. Kramer and J. R. Evans, Plant Physiol., 2011, 155, 70–78 CrossRef CAS PubMed.
- A. Montagud, E. Navarro, P. Fernandez de Cordoba, J. F. Urchueguia and K. R. Patil, BMC Syst. Biol., 2010, 4, 156 CrossRef PubMed.
- J. H. Park, K. H. Lee, T. Y. Kim and S. Y. Lee, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 7797–7802 CrossRef CAS PubMed.
- D. R. Hyduke, N. E. Lewis and B. O. Palsson, Mol. BioSyst., 2013, 9, 167–174 RSC.
- D. McCloskey, B. O. Palsson and A. M. Feist, Mol. Syst. Biol., 2013, 9, 661 CrossRef CAS PubMed.
- A. A. Shastri and J. A. Morgan, Biotechnol. Prog., 2005, 21, 1617–1626 CrossRef CAS PubMed.
- K. Yoshikawa, et al. , Appl. Microbiol. Biotechnol., 2011, 92, 347–358 CrossRef CAS PubMed.
- P. Fu, J. Chem. Technol. Biotechnol., 2009, 84, 473–483 CrossRef CAS.
- R. Saha, et al. , PLoS One, 2012, 7, e48285 CAS.
- H. Knoop, Y. Zilliges, W. Lockau and R. Steuer, Plant Physiol., 2010, 154, 410–422 CrossRef CAS PubMed.
- T. T. Vu, et al. , Biotechnol. J., 2013, 8, 619–630 CrossRef CAS PubMed.
- E. Vitkin and T. Shlomi, Genome Biol., 2012, 13, R111 CrossRef PubMed.
- L. You, B. Berla, L. He, H. B. Pakrasi and Y. J. Tang, Biotechnol. J., 2014, 9, 684–692 CrossRef CAS PubMed.
- W. Xiong, D. Brune and W. F. J. Vermaas, Mol. Microbiol., 2014, 93, 786–796 CrossRef CAS PubMed.
- R. Saha, A. Chowdhury and C. D. Maranas, Curr. Opin. Biotechnol., 2014, 29C, 39–45 CrossRef PubMed.
- A. Montagud, et al. , Biotechnol. J., 2011, 6, 330–342 CrossRef CAS PubMed.
- A. K. Singh, et al. , BMC Syst. Biol., 2010, 4, 105 Search PubMed.
- A. P. Burgard, P. Pharkya and C. D. Maranas, Biotechnol. Bioeng., 2003, 84, 647–657 CrossRef CAS PubMed.
- C. Cotten and J. L. Reed, Biotechnol. J., 2013, 8, 595–604 CrossRef CAS PubMed.
- A. M. Feist, et al. , Metab. Eng., 2010, 12, 173–186 CrossRef CAS PubMed.
- S. Ranganathan, P. F. Suthers and C. D. Maranas, PLoS Comput. Biol., 2010, 6, e1000744 Search PubMed.
- M. H. Medema, R. van Raaphorst, E. Takano and R. Breitling, Nat. Rev. Microbiol., 2012, 10, 191–202 CrossRef CAS PubMed.
- J. Nogales, S. Gudmundsson and I. Thiele, Bioengineered, 2013, 4, 158–163 CrossRef PubMed.
- R. Steuer, H. Knoop and R. Machne, J. Exp. Bot., 2012, 63, 2259–2274 CrossRef CAS PubMed.
- R. H. Wijffels, O. Kruse and K. J. Hellingwerf, Curr. Opin. Biotechnol., 2013, 24, 405–413 CrossRef CAS PubMed.
- R. L. Chang, et al. , Mol. Syst. Biol., 2011, 7, 518 CrossRef PubMed.
- P. Pharkya, A. P. Burgard and C. D. Maranas, Genome Res., 2004, 14, 2367–2376 CrossRef CAS PubMed.
- P. E. Savakis, S. A. Angermayr and K. J. Hellingwerf, Metab. Eng., 2013, 20, 121–130 CrossRef CAS PubMed.
- Z. A. King and A. M. Feist, Metab. Eng., 2014, 24, 117–128, DOI:10.1016/j.ymben.2014.05.009.
- S. D. Finley, L. J. Broadbelt and V. Hatzimanikatis, Biotechnol. Bioeng., 2009, 104, 1086–1097 CrossRef CAS PubMed.
- Y. Moriya, et al. , Nucleic Acids Res., 2010, 38, W138–W143 CrossRef CAS PubMed.
- T. M. Conrad, N. E. Lewis and B. Ø. Palsson, Mol. Syst. Biol., 2011, 7, 509 CrossRef PubMed.
- J. Monk, J. Nogales and B. O. Palsson, Nat. Biotechnol., 2014, 32, 447–452 CrossRef CAS PubMed.
- E. Goncalves, et al. , Mol. BioSyst., 2013, 9, 1576–1583 RSC.
- J. A. Lerman, et al. , Nat. Commun., 2012, 3, 929 CrossRef PubMed.
- I. Thiele and B. O. Palsson, Nat. Protoc., 2010, 5, 93–121 CrossRef CAS PubMed.
- N. M. O'Boyle, et al. , J. Cheminf., 2011, 3, 33 Search PubMed.
- L. J. P. v. d. Maaten and G. E. Hinton, J. Mach. Learn. Res., 2008, 9, 2579–2605 Search PubMed.
- J. D. Orth, et al. , Mol. Syst. Biol., 2011, 7, 535 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4mb00335g |
| This journal is © The Royal Society of Chemistry 2015 |