Open Access Article
Open Access ArticlePhotopatterned oil-reservoir micromodels with tailored wetting properties†
Hyundo
Lee
a,
Seung Goo
Lee
b and
Patrick S.
Doyle
*b
aDepartment of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA
bDepartment of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA. E-mail: pdoyle@mit.edu; Fax: +1 617 258 5042; Tel: +1 617 253 4534
First published on 9th June 2015
Abstract
Micromodels with a simplified porous network that represents geological porous media have been used as experimental test beds for multiphase flow studies in the petroleum industry. We present a new method to fabricate reservoir micromodels with heterogeneous wetting properties. Photopatterned, copolymerized microstructures were fabricated in a bottom-up manner. The use of rationally designed copolymers allowed us to tailor the wetting behavior (oleophilic/phobic) of the structures without requiring additional surface modifications. Using this approach, two separate techniques of constructing microstructures and tailoring their wetting behavior are combined in a simple, single-step ultraviolet lithography process. This microstructuring method is fast, economical, and versatile compared with previous fabrication methods used for multi-phase micromodel experiments. The wetting behaviors of the copolymerized microstructures were quantified and demonstrative oil/water immiscible displacement experiments were conducted.
1 Introduction
It is common practice to use core samples from oil reservoir sites to understand oil–gas–water multiphase flow occurring in underground oil reservoirs.1–7 However, the disadvantages of core-flooding experiments (e.g. opacity, site specificity, ambiguities of experimental parameters) have impeded fundamental investigations of oil reservoirs in the laboratory environment.Due to such limitations of core-flooding experiments, researchers have recently developed synthetic micromodels using microfabrication techniques. Micromodels are usually two-dimensional and transparent microchannels with a simplified porous network designed to visualize and study fluid behavior in porous media.8–10 In oil reservoir research, micromodels reflect underground oil reservoir conditions, for example, porosity, permeability, and wettability.11–14 These reservoir properties are designed and built into micromodels for further understanding of fundamental fluid behavior and interactions among oil–water–rock phases. Micromodel studies in the laboratory environment are required for various real field applications, such as operational practices for oil production, enhanced oil recovery with various materials (surfactant,15 polymer,16 foam,17,18 steam19), and reservoir network mapping.20
It is estimated that more than 60% of the world's oil and 40% of the world's gas reserves are held in carbonate reservoirs. For example, the Middle East is dominated by carbonate rocks, with around 70% of oil and 90% of gas reserves held within these reservoirs. When it comes to carbonate reservoirs, even though their main component, calcite, inherently shows water-wet behavior, the surface wetting property of carbonate oil reservoir rocks is known to be mostly oil-wet.21 Treiber et al. investigated 50 samples and showed that only 8% of the carbonate reservoir rocks were water-wet, 8% intermediate-wet, and 84% oil-wet.22 That being said, the pertinent wetting properties of micromodels were not measured or controlled in previous studies. Most prior research only investigated the wettability of micromodels in solid–liquid–air systems, hydro-philicity/phobicity in air or oleo-philicity/phobicity in air. Since both hydrophilic and hydrophobic surfaces might be oleophilic (or oleophobic) in water,23 it is necessary to investigate the micromodel's wetting preference in the presence of both water and oil.23–25
Various fabrication techniques have been employed to build micromodels,8,9 including plasma etching of silicon,26 deep reactive ion etching of glass,27,28 and soft lithography with polymeric materials.17,29–37 More recently, Song et al. developed a real-rock micromodel by wet etching of natural carbonate rock.38 These fabrication methods are often time consuming and involve access to cleanroom microfabrication facilities which can result in high unit costs for a single micromodel. Moreover, once the design of features or patterns are fabricated, it is nearly impossible to make geometric modifications on-the-fly and quickly iterate on designs.
In addition to micromodel fabrication methods, there have been significant advances in methods to modify wetting properties within micromodels to mimic an oil reservoir's wetting features. These approaches include silanization,39 UV-ozone treatment,17,40 and UV-initiated polymer grafting.31,41 However, these approaches to tune wettability also have some limitations. They all require additional post-processing of a device. Furthermore it is well known that an UV-ozone treated PDMS (polydimethylsiloxane) surface recovers its native hydrophobicity by the oligomer migration from the bulk PDMS to the surface.42,43 Moreover, due to the lack of ability to tune wetting over a continuous range, it is hard to reproduce the complex wetting properties of an oil reservoir having locally different and more than two surface properties. A more detailed comparison of micromodel fabrication and tailored wettability approaches is given in the ESI.† To summarize, the major shortcomings of previous fabrication methods for oil-reservoir micromodels are inflexibility in design and the lack of control in wetting properties.
By adapting the Stop-Flow-Lithography (SFL) technique,44–46 we have developed a new, versatile, and bottom-up micromodel fabrication technique in which microstructure synthesis and wetting property control are combined in a single lithographic step. Unlike SFL, which is used to create free-standing microparticles, here the top and bottom glass surfaces of microchannels are acrylated, and so the polymerized microstructures adhere to the glass. We demonstrate that quasi-two-dimensional porous structures with defined geometric features and predetermined heterogeneous wetting properties can be polymerized within seconds.
2 Materials and methods
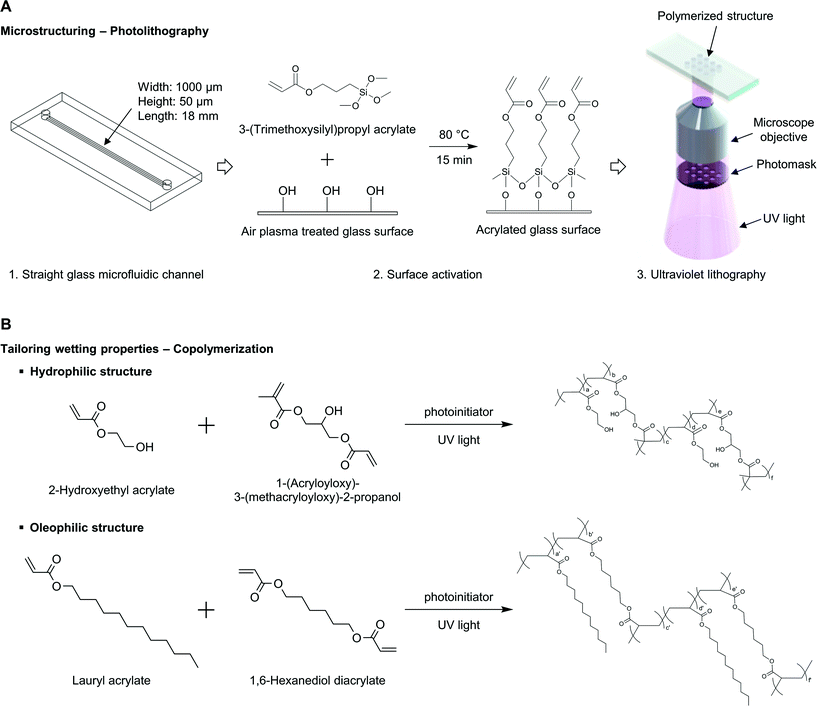
In this work, we utilized the stop-flow-lithography (SFL) technique45 to construct polymeric structures in initially empty microchannels. SFL introduces streams of precursor with a controlled-pressure pump, stops the flow, then polymerizes microparticles with specific geometric features through pre-defined transparent masks, and finally releases microparticles with supplying pressure again. This stop-polymerization-flow sequence is repeated, and SFL produces high-resolution microparticles at high throughput. Instead of producing functional microparticles, we utilized this photopatterning technique to build polymeric structures in initially empty microchannels.2.1 Surface activation for polymeric structure fixation
During the SFL process, polymerized particles are not bound to the top and bottom surfaces due to the oxygen permeability of PDMS (polydimethylsiloxane); thin oxygen layers inhibit the polymerization of precursor solutions near top and bottom surfaces.47 Since we wanted to build desired structures in microchannels using a bottom-up approach, it was necessary to either control the oxygen environment during polymerization or use gas-impermeable surfaces; we chose the latter. It is also necessary to modify the chemistry of top and bottom surfaces to hold the polymerized structures in place for future use of flow studies. We adapted the following surface activation procedure as previously done by Srinivas et al. for fixing PEG-DA (poly(ethylene glycol)diacrylate) hydrogel posts in bioassays.48First an empty glass microfluidic channel (Hilgenberg GmbH, Germany) was filled with 1 M sodium hydroxide aqueous solution for 1 hour. After thoroughly rinsing the microchannel with ethanol and water, the channel was filled with 3-(trimethoxysilyl)propyl acrylate (Sigma-Aldrich) for 5 minutes. The microchannel was thoroughly rinsed with ethanol and water again, and cured at 80 °C for 15 minutes for sufficient reaction. Later, when ultraviolet polymerization reaction occurred, the top and bottom glass surfaces modified with acrylate functional group promoted polymer structure fixation on the surfaces (Fig. 1A).
2.2 Microstructuring by photolithography
With proper ratios of crosslinkers, monomers, and a photoinitiator, hydrophilic and oleophilic structures were built into surface activated glass microchannels. A thoroughly mixed precursor solution was injected into the activated glass–glass channel and UV-polymerization was done on the stage of an inverted microscope (Zeiss Axio Observer A1). A photomask of desired geometric shape was inserted into the field stop of the microscope, and the microchannel was then placed and aligned properly. UV exposure time was controlled by switching a LED light source on and off with LabView. After building microstructures, any uncured precursor solution was washed out of the device with acetone (Fig. 1A).2.3 Tailoring wetting properties by copolymerization
The displacement of oil with other fluids that exist in reservoirs (e.g. gas, brine, water), needs to be considered at the pore scale. When the transport phenomena occur in porous reservoirs, the wetting property is a critical factor and has to be taken into consideration, as well as other geometric features such as porosity and permeability. In this experimental study, we are interested in oil–water flow around geometric structures with tuned wetting properties. As mentioned previously, the relative preference between oil and water has to be precisely defined and investigated in water–oil displacement study to understand displacement process occurring underground oil reservoir. Since the wettability is a relative preference of a solid surface to different fluids, it is prerequisite to have designed wetting preferences of oil-like (oleophilic) and water-like (hydrophilic) depending on the configuration of water contact angle in oil.49 The concept of hydrophilic/hydrophobic is usually defined in the presence of solid–water–air, however that of oleophilic/oleophobic is inarticulately used, it is used to define the relation of solid–oil–air or solid–water–oil. A common mistake is to assume that a hydrophobic surface is then oleophilic. A hydrophobic surface having a high water contact angle in the air does not prefer water to air; however, this does not necessarily mean that the surface will like oil than water. In other words, when the wetting property of a surface is investigated, one has to check its contact angle in the presence of the both of immiscible fluids (solid–water–oil) or has to check the water contact angle in air and the oil contact angle in air separately, and then correlate the relationship of solid–water–oil. This relationship between hydrophilic/phobic and oleophilic/phobic is clearly explained in the article of Jung and Bhushan.23 For these reasons, in our study we measured water contact angles in decane to define the polymerized surfaces' relative preference between oil and water. Throughout this article, we use terms hydrophilic and oleophilic expressing a solid surface's behavior of water-like and oil-like, respectively, in the presence of oil and water.Our approach to tailoring the wetting property of polymeric structures is through tuning the ratio of hydrophilic and oleophilic components. Here, we use polymeric crosslinkers that have reactive vinyl groups, which can polymerize with photoinitiator, 2-hydroxy-2-methylpropiophenon (Darocur 1173, Sigma-Aldrich), upon exposure to the ultraviolet light. We choose hydrophilic and oleophilic crosslinking agents, 1-(acryloyloxy)-3-(methacryloyloxy)-2-propanol (AMP) (Polysciences, Inc.) and 1,6-hexanediol diacrylate (HDDA) (Sigma-Aldrich), respectively, that can confer chemical and physical robustness. The desired wetting property is further increased by copolymerization with additives, hydrophilic and oleophilic monomers, 2-hydroxyethyl acrylate (HEA) (Sigma-Aldrich) and lauryl acrylate (LA) (Sigma-Aldrich) (Fig. 1B, Table 1).
| Hydrophilic | Oleophilic | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H-1 | H-2 | H-3 | H-4 | H-5 | O-1 | O-2 | O-3 | O-4 | O-5 | ||
| 1-(Acryloyloxy)-3-(methacryloyloxy)-2-propanol | 15% | 35% | 55% | 75% | 95% | 1,6-Hexanediol diacrylate | 15% | 35% | 55% | 75% | 95% |
| 2-Hydroxyethyl acrylate | 80% | 60% | 40% | 20% | 0% | Lauryl acrylate | 80% | 60% | 40% | 20% | 0% |
| Darocur 1173 | 5% | 5% | 5% | 5% | 5% | Darocur 1173 | 5% | 5% | 5% | 5% | 5% |
3 Result and discussion
3.1 Water contact angle measurement in decane
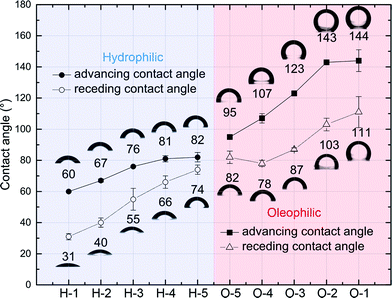
Hydrophilic and oleophilic substrates with controlled crosslinker-monomer volume–volume percent concentration were prepared by pressing a drop of the precursor solution on an acrylated glass. The solution was pressed with another glass slide, and then it was cured under the UV (365 nm) lamp for 3 minutes. The water contact angle on the substrate was measured in a quartz cell which is filled with decane using a goniometer (Rame-Hart). Each measurement of advancing and receding contact angles of water in decane was repeated three times. More details of the contact angle measurement process is described in ESI†.Fig. 2 shows the trend of water contact angles in decane with respect to crosslinker-monomer volume–volume percent concentration of hydrophilic and oleophilic precursors (Table 1). We modulated the wetting properties of substrates from hydrophilic to oleophilic in a continuous range (θadvwater in oil from 60° to 144°) by tuning the ratio of components. As the portion of the hydrophilic or oleophilic additive (HEA or LA) increased, the hydroxyl or hydrocarbon of content of the polymer surfaces showed stronger interactions with polar (water) and nonpolar (decane) phases, that decreased and increased the water contact angle, respectively.
As the ratio of hydrophilic or oleophilic functionalities increase, contact angle hysteresis, the difference between advancing and receding contact angles, is also increased (H-5 → H-1, O-5 → O-1). This contact angle hysteresis increase is most likely due to the change of crosslinking density, as mentioned in as previous studies that investigated contact angles on polymer surfaces.50–52 Less crosslinked polymeric surfaces are facile to rearrange their chemical structure upon the wetting of solvent, and thus allow the solvent penetration. However, highly crosslinked surfaces inhibit this restructuring by constrained chain mobility, and the solvent is unable to penetrate. As a result, the surfaces of lower crosslinking density have the larger difference of the surface energy for the polar and nonpolar solvents wetting, consequently show higher contact angle hysteresis.51
3.2 Immiscible fluid displacement – single post
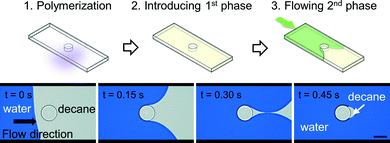
After polymerization of microstructures in empty microchannels and thoroughly rinsing uncured precursor solutions with acetone, subsequent flow experiments were conducted (Fig. 3). The micromodel was then filled with decane, and water displaced decane under constant applied pressure (Fig. 4A). Experiments with a reversed flow sequence were also performed (Fig. 4B). A movie and sequentially captured images showing the displacement process for the oleophilic post (O-1) is available as ESI.†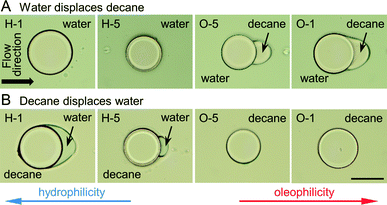 | ||
| Fig. 4 (A) Microchannels were first filled with decane, and then water was introduced into the channels (flow direction from the left to the right). The alphanumeric code refers to the copolymer compositions listed in Table 1, H-1 being the most hydrophilic and O-1 the most oleophilic. Water can completely displace decane for the hydrophilic post systems (H-1, H-5), while decane remains entrained by the oleophilic posts (O-5, O-1). The most oleophilic post (O-1) is completely encapsulated by decane, while the less oleophilic post (O-5) has a well-defined droplet adhered to the downstream side. (B) Flow sequence is reversed relative to (A): the channels were filled with water, and then water was displaced by decane. Similar to the previous displacement, the most hydrophilic post (H-1) was entirely surrounded by water, H-5 was partially covered with water, and no water reamained around the oleophilic posts. Scale bar is 100 μm. | ||
Since the oleophilic posts (O-1, O-5) prefer oil to water, the oleophilic post formed a thin oil layer between the posts and water. The most oleophilic post (O-1) was eventually encapsulated by oil in the case of oil → water flow, while the hydrophilic posts (H-1, H-5) left water at the posterior area in the case of water → oil flow. In the opposite flow sequence for each post, water/oil was repelled by oleophilic/hydrophilic posts since the posts prefer the displacing phase to displaced phase. Computational simulation of the fluid displacement for single posts was performed in OpenFOAM 2.3.0 and the results are available as ESI.†
Based on these initial experiment sets, we can construct a set of eight possible experimental combinations: four posts of different wetting preferences and two flow sequences (oil → water, water → oil) (Fig. 4). These eight combinations can be used to demonstrate typical underground reservoir scenarios: the drainage process (when a non-wetting entering fluid displaces a wetting fluid) and the imbibition process (when a wetting fluid displaces a non-wetting fluid).
Four posts in Fig. 4 were photopatterned with the same photomask, thus right after polymerization all four posts had the same diameters. However, there were slight size changes at different solvent conditions. There was no significant size changes with homogeneous hydrophilic (H-5, AMP 95%, photoinitiator 5%) and oleophilic (O-5, HDDA 95%, photoinitiator 5%) posts. On the other hand, the most hydrophilic (H-1, AMP 15%, HEA 80%, photoinitiator 5%) and oleophilic (O-1, HDDA 15%, LA 80%, photoinitiator 5%) posts showed different sizes at different solvent conditions (acetone, water, and decane). This different swelling behavior can be explained with the same reason of previous contact angle hysteresis trend, the crosslinking density and the affinity of hydrophilic/oleophilic posts for polar/nonpolar solvents.51–55 In comparison with H-5 or O-5, H-1 and O-1 had relatively lower crosslinker and higher hydrophilc/oleophilic monomer concentrations. As a result, the higher molecular chain mobility and the larger mesh size increased restructuring ability and allowed polar/nonpolar solvent penetration, thus the posts were swelled. The post size changes in different solvents was investigated, and the result is shown in the accompanying ESI.†
3.3 Multi-post micromodel with heterogeneous wettability
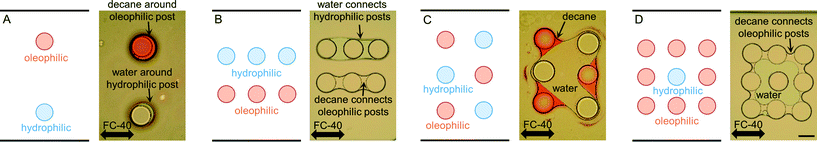
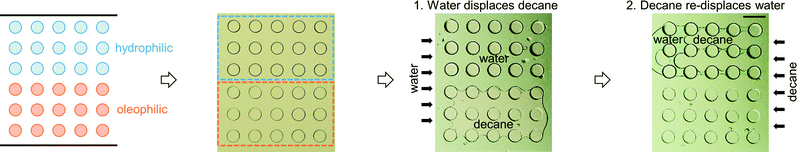
The first multi-post micromodel was created by polymerizing multiple posts one by one. The hydrophilic posts had been created, and the channel was rinsed, then the oleophilic posts were polymerized with the same photomask (Fig. 5). Immiscible fluids were sequentially injected into the channel at constant pressures (water → decane → fluorinated oil, FC-40; immiscible to both water and decane).Decane (dyed with an oil dye, Oil Red O (Sigma-Aldrich)), was held on the oleophilic post (red-stained with the oil dye), and water was captured on the hydrophilic post (Fig. 5A). Water and decane pathways were formed in parallel following each set of three hydrophilic and oleophilic posts (Fig. 5B), and a V-shape water reservoir was made (Fig. 5C). A square shaped water reservoir was isolated from the FC-40 and was surrounded by decane (Fig. 5D). Because the hydrophilic and oleophilic posts were already occupied with their preferred phases, FC-40 was not able to displace the decane and water being entrained by the posts.
The second multi-post micromodel was constructed with a photomask having multiple geometric features, in this case a 3 × 5 array of circles. We made separate hydrophilic (H-1) and oleophilic (O-1) areas with two different precursors. After thoroughly rinsing with acetone, the microchannel was first filled with decane, displaced by water from the left to the right, and displaced again by decane from the right to the left to see different behaviors (Fig. 6). From the first displacement image, we can see that decane was fully displaced by water in the hydrophilic region while water was not able to penetrate through the oleophilic region (Fig. 6-1). To confirm the hydrophilicity of the upper half, decane was re-introduced from the right side of the image, and it is clearly shown that hydrophilic posts were connected by water along the direction of the flow (Fig. 6-2).
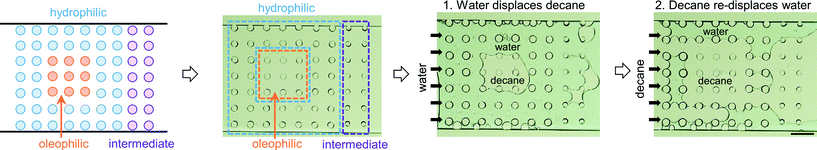
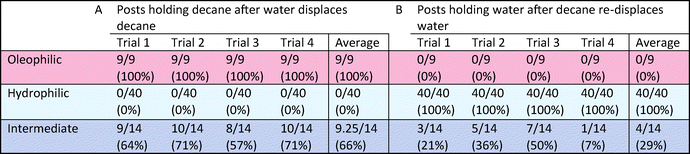
The third multi-post micromodel had three different regions of wetting. The ability to tune wettability in a continuous manner is one of the advantages of our approach compared with other methods. Typically other wettability modification methods can achieve only binary behavior (mostly hydrophilic or hydrophobic). Three different precursors were used to create three different wetting regions (hydrophilic (H-1), oleophilic (O-1), and intermediate (O-5)), and decane and water were introduced into the micromodel (Fig. 7). After decane displacement by water, all of 9 oleophilic posts (O-1) held decane while decane was totally displaced by water at all of the 40 hydrophilic posts (H-1). Around 9 intermediate posts (O-5) out of 14 retained decane around them after water flooding. When decane was re-introduced, the opposite result was obtained: none of the 9 oleophilic posts (O-1) held water, and all of the 40 hydrophilic posts (H-1) formed a water film around which isolated them from decane. Around 3 intermediate posts (O-5) were wetted by the displaced water. These trends are expected based on the results of previously described single post experiment. For the multi-post microfluidic channel used in Fig. 7, we repeated the same decane/water displacement experiments 4 times with the same device, and observed that the oleophilic (O-1) and hydrophilic posts (H-1) always showed the same result of totally wetting and totally non-wetting with respect to their preferred and unpreferred phases (Fig. 8, Table 2).
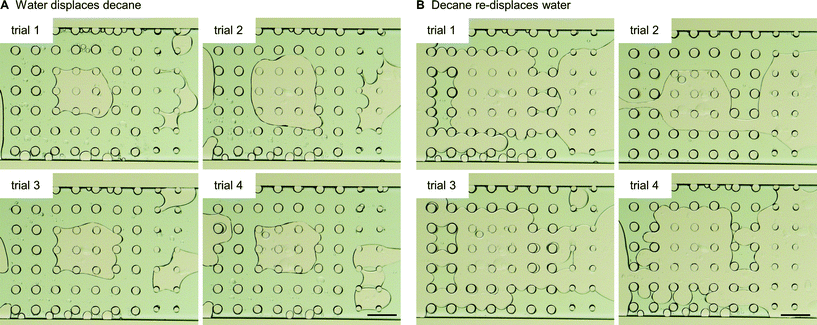 | ||
| Fig. 8 For the same multi-post microfluidic channel used in Fig. 7, we repeated the same decane/water displacement experiments, and counted how many posts held the displaced phase after the other phase was introduced. Images after (A) water displaces decane, and (B) decane re-displaces water. Scale bar is 200 mm. | ||
Based on the above three experiments, it is possible to have insight into how the porosity (the fraction of void space) and the wettability (the relative preference of solid phase for fluid phases) influence the displacement process. It is well known by Darcy's law that the lower the porosity, the lower the permeability. However, as can be seen through the above experiments, we can infer that even for the same porosity, the drainage process requires higher pressure than the imbibition process. During the drainage process (when most of the posts prefer the displaced phase to the displacing phase), the higher pressure is needed to displace the wetting phase. On the other hand, during the imbibition process (when the posts prefer the displacing phase to the displaced phase), the non-wetting phase is easily displaceable since the posts prefer the displacing phase to the displaced phase. In other words, wettability plays a role in immiscible displacement as important as the porosity does. Moreover, the influence of wettability may increase as effective surface area increases, such as the case of smaller posts having the same porosity. With further experiments using multi-post micromodels, we can broaden our understanding of how the geometric and the chemical properties of a micromodel are correlated to and affect the displacement process.
4 Conclusions
In this study, we have introduced a new on-demand micromodel fabrication technique with tailored wetting properties. Unlike conventional soft lithography, our approach does not require a master silicon wafer to be prepared in a clean room, nor does it require significant time and effort to fabricate different porous geometries and to modify wetting properties of the micromodels. Geometric features, such as the shape, size, and distance between microstructures can be controlled for micromodel design optimization or systematical study of their effects. At the same time, each structure can have a different wetting property over a continuous range that is pre-formulated, without additional surface modification steps. This is the first method which can simultaneously create and tailor the wettability of a microstructure in a fluidic device. We demonstrated experiments on immiscible fluid displacement with single posts. We also demonstrated oil–water displacement studies in devices with spatially varying wetting properties. Like real reservoir formations, we can mimic locally oleophilic niches within an otherwise hydrophilic channel. To the best of our knowledge, this is also the first micromodel that has more than a binary wettability within it.Micromodels have broadened our understanding of multi-phase flow in porous media and have provided solutions for practical engineering issues by visualizing phenomena occurring in subsurface oil reservoirs. However, multi-phase flow mechanisms at the pore scale are still not clearly explainable, and newly developed practices or functional materials need repeated experiments before applying them at production sites. In this context, the micromodel fabrication method demonstrated in this article can be used for rapid prototyping. To understand fundamental physics of underground flows, it is more desirable to start with a micromodel that has simple and prescribed structures, rather than injecting immiscible fluids into the black-box micromodel that has complex geometries and undefined wettability. New production practices and materials can be tested within well-defined micromodels that can represent various types of oil reservoirs.
In addition to the oil-reservoir studies, this fabrication method can be utilized for other applications in microfluidics. For example, one can use our method to rapidly prototype pillar arrangements within a flow device that can then be used to sculpt complex chemical patterns.56 The controlled wetting within a device can be useful to stabilize fluid-fluid interfaces as particles are pulled across them by magnetic forces for separations.57 Internal features with controlled wetting will also be useful in capillary guided wetting processes58 for assembly of cells and organ on chip devices. From the technical side, the microfluidic device production can be improved by a stand-alone, photopatterning setup which has a broader UV lithography area or by a microscope with an automated positioning stage for a precisely controlled configuration of microstructures.
Acknowledgements
We acknowledge Ankur Gupta for help on the computational simulation. This work was supported by funding from Saudi Aramco and the NSF grants DMR-1006147 and CMMI-1120724.References
- C. C. Mattax and J. R. Kyte, Soc. Pet. Eng. J., 1962, 2, 177–184 CrossRef PubMed
.
- W. W. Owens and D. L. Archer, J. Pet. Technol., 1971, 23, 873–878 CrossRef PubMed
.
- R. A. Salathiel, J. Pet. Technol., 1973, 25, 1216–1224 CrossRef PubMed
.
- A. R. Kovscek, H. Wong and C. J. Radke, AIChE J., 1993, 39, 1072–1085 CrossRef CAS PubMed
.
- J.-C. Perrin and S. Benson, Transp. Porous Media, 2010, 82, 93–109 CrossRef CAS
.
- T. Austad, S. F. Shariatpanahi, S. Strand, C. J. J. Black and K. J. Webb, Energy Fuels, 2012, 26, 569–575 CrossRef CAS
.
- S. C. M. Krevor, R. Pini, L. Zuo and S. M. Benson, Water Resour. Res., 2012, 48(2) DOI:10.1029/2011WR010859
.
- N. K. Karadimitriou and S. M. Hassanizadeh, Vadose Zone J., 2012, 11(3) DOI:10.2136/vzj2011.0072
.
- C. Tsakiroglou, O. Vizika-kavvadias and R. Lenormand, Int. Symp. Soc. Core Anal., 2013, pp. 1–13 Search PubMed.
- D. Sinton, Lab Chip, 2014, 14, 3127–3134 RSC
.
- C. U. Hatiboglu and T. Babadagli, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2008, 77, 066311 CrossRef
.
- C. Cottin, H. Bodiguel and A. Colin, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2011, 84, 026311 CrossRef
.
- C. Zhang, M. Oostrom, T. W. Wietsma, J. W. Grate and M. G. Warner, Energy Fuels, 2011, 25, 3493–3505 CrossRef CAS
.
- J. Joseph, N. Siva Kumar Gunda and S. K. Mitra, Chem. Eng. Sci., 2013, 99, 274–283 CrossRef CAS PubMed
.
- K. He, L. Xu, Y. Gao, K. B. Neeves, X. Yin, B. Bai, Y. Ma and J. Smith, Pap. SPE 169147, 2014, pp. 12–16 Search PubMed.
- J. Beaumont, H. Bodiguel and A. Colin, Soft Matter, 2013, 9, 10174–10185 RSC
.
- K. Ma, R. Liontas, C. A. Conn, G. J. Hirasaki and S. L. Biswal, Soft Matter, 2012, 8, 10669 RSC
.
- C. A. Conn, K. Ma, G. J. Hirasaki and S. L. Biswal, Lab Chip, 2014, 14, 3968–3977 RSC
.
- T. W. Haas, H. Fadaei, U. Guerrero and D. Sinton, Lab Chip, 2013, 13, 3832–3839 RSC
.
- G. C. Kini, J. Yu, L. Wang, A. T. Kan, S. L. Biswal, J. M. Tour, M. B. Tomson and M. S. Wong, Colloids Surf., A, 2014, 443, 492–500 CrossRef CAS PubMed
.
- T. M. Okasha, J. J. Funk and H. N. Rashidi, SPE Middle East Oil Gas Show Conf., 2007, pp. 1–10 Search PubMed.
- L. E. Treiber and W. W. Owens, Soc. Pet. Eng. J., 1972, 12, 531–540 CrossRef PubMed
.
- Y. C. Jung and B. Bhushan, Langmuir, 2009, 25, 14165–14173 CrossRef CAS PubMed
.
- M. Jin, J. Wang, X. Yao, M. Liao, Y. Zhao and L. Jiang, Adv. Mater., 2011, 23, 2861–2864 CrossRef CAS PubMed
.
- A. K. Kota, G. Kwon, W. Choi, J. M. Mabry and A. Tuteja, Nat. Commun., 2012, 3, 1025 CrossRef PubMed
.
- N. S. K. Gunda, B. Bera, N. K. Karadimitriou, S. K. Mitra and S. M. Hassanizadeh, Lab Chip, 2011, 11, 3785–3792 RSC
.
- J. W. Grate, R. T. Kelly, J. Suter and N. C. Anheier, Lab Chip, 2012, 12, 4796–4801 RSC
.
- N. K. Karadimitriou, V. Joekar-Niasar, S. M. Hassanizadeh, P. J. Kleingeld and L. J. Pyrak-Nolte, Lab Chip, 2012, 12, 3413–3418 RSC
.
- D. Bartolo, G. Degré, P. Nghe and V. Studer, Lab Chip, 2008, 8, 274–279 RSC
.
- V. Berejnov, N. Djilali and D. Sinton, Lab Chip, 2008, 8, 689–693 RSC
.
- M. H. Schneider and P. Tabeling, Am. J. Appl. Sci., 2011, 8, 927–932 CrossRef
.
- E. Sollier, C. Murray, P. Maoddi and D. Di Carlo, Lab Chip, 2011, 11, 3752–3765 RSC
.
- M. Wu, F. Xiao, R. M. Johnson-Paben, S. T. Retterer, X. Yin and K. B. Neeves, Lab Chip, 2012, 12, 253–261 RSC
.
- K. W. Bong, J. Xu, J.-H. Kim, S. C. Chapin, M. S. Strano, K. K. Gleason and P. S. Doyle, Nat. Commun., 2012, 3, 805 CrossRef PubMed
.
- P. Horgue, F. Augier, P. Duru, M. Prat and M. Quintard, Chem. Eng. Sci., 2013, 102, 335–345 CrossRef CAS PubMed
.
- N. K. Karadimitriou, M. Musterd, P. J. Kleingeld, M. T. Kreutzer, S. M. Hassanizadeh and V. Joekar-Niasar, Water Resour. Res., 2013, 49, 2056–2067 CrossRef PubMed
.
- Q. Zhang, N. K. Karadimitriou, S. M. Hassanizadeh, P. J. Kleingeld and A. Imhof, J. Colloid Interface Sci., 2013, 401, 141–147 CrossRef CAS PubMed
.
- W. Song, T. W. de Haas, H. Fadaei and D. Sinton, Lab Chip, 2014, 14, 4382–4390 RSC
.
- J. W. Grate, M. G. Warner, J. W. Pittman, K. J. Dehoff, T. W. Wietsma, C. Zhang and M. Oostrom, Water Resour. Res., 2013, 49, 4724–4729 CrossRef CAS PubMed
.
- B. Levaché, A. Azioune, M. Bourrel, V. Studer and D. Bartolo, Lab Chip, 2012, 12, 3028–3031 RSC
.
- M. H. Schneider, H. Willaime, Y. Tran, F. Rezgui and P. Tabeling, Anal. Chem., 2010, 82, 8848–8855 CrossRef CAS PubMed
.
- H. Hillborg, N. Tomczak, A. Ola, H. Scho and G. J. Vancso, Langmuir, 2004, 20, 785–794 CrossRef CAS
.
- A. Oláh, H. Hillborg and G. Vancso, Appl. Surf. Sci., 2005, 239, 410–423 CrossRef PubMed
.
- D. Dendukuri, D. C. Pregibon, J. Collins, T. A. Hatton and P. S. Doyle, Nat. Mater., 2006, 5, 365–369 CrossRef CAS PubMed
.
- D. Dendukuri, S. S. Gu, D. C. Pregibon, T. A. Hatton and P. S. Doyle, Lab Chip, 2007, 7, 818–828 RSC
.
- K. W. Bong, D. C. Pregibon and P. S. Doyle, Lab Chip, 2009, 9, 863–866 RSC
.
- D. Dendukuri, P. Panda, R. Haghgooie, J. M. Kim, T. A. Hatton and P. S. Doyle, Macromolecules, 2008, 41, 8547–8556 CrossRef CAS
.
- R. L. Srinivas, S. D. Johnson and P. S. Doyle, Anal. Chem., 2013, 85, 12099–12107 CrossRef CAS PubMed
.
- Z. Cheng, H. Lai, Y. Du, K. Fu, R. Hou, N. Zhang and K. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 11363–11370 CAS
.
- H. Yasuda, A. K. Sharma and T. Yasuda, J. Polym. Sci., Polym. Phys. Ed., 1981, 19, 1285–1291 CrossRef CAS PubMed
.
- D. L. Schmidt, R. F. Brady, K. Lam, D. C. Schmidt and M. K. Chaudhury, Langmuir, 2004, 20, 2830–2836 CrossRef CAS
.
- J. C. Yarbrough, J. P. Rolland, J. M. Desimone, M. E. Callow, J. A. Finlay and J. A. Callow, Macromolecules, 2006, 39, 2521–2528 CrossRef CAS
.
- S. J. Kim, S. J. Park and S. I. Kim, React. Funct. Polym., 2003, 55, 53–59 CrossRef CAS
.
- D. K. Hwang, J. Oakey, M. Toner, J. A. Arthur, K. S. Anseth, S. Lee, A. Zeiger, K. J. V. Vliet and P. S. Doyle, J. Am. Chem. Soc., 2009, 131, 4499–4504 CrossRef CAS PubMed
.
- Y. Diao, K. E. Whaley, M. E. Helgeson, M. A. Woldeyes, P. S. Doyle, A. S. Myerson, T. A. Hatton and B. L. Trout, J. Am. Chem. Soc., 2012, 134, 673–684 CrossRef CAS PubMed
.
- H. Amini, E. Sollier, M. Masaeli, Y. Xie, B. Ganapathysubramanian, H. A. Stone and D. Di Carlo, Nat. Commun., 2013, 4, 1826 CrossRef PubMed
.
- S. S. H. Tsai, J. S. Wexler, J. Wan and H. A. Stone, Appl. Phys. Lett., 2011, 99, 19–22 Search PubMed
.
- M. Kang, W. Park, S. Na, S.-M. Paik, H. Lee, J. W. Park, H.-Y. Kim and N. L. Jeon, Small, 2015, 11, 2789–2797 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5lc00277j |
| This journal is © The Royal Society of Chemistry 2015 |