Pyrolysis reaction networks for lignin model compounds: unraveling thermal deconstruction of β-O-4 and α-O-4 compounds†
Abstract
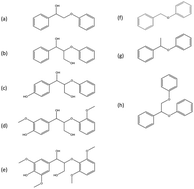
Although lignin is one of the main components of biomass, its pyrolysis chemistry is not well understood due to complex heterogeneity. To gain insights into this chemistry, the pyrolysis of seven lignin model compounds (five β-O-4 and two α-O-4 linked molecules) was investigated in a micropyrolyzer connected to GC-MS/FID. According to quantitative product mole balance for the reaction networks, concerted retro–ene fragmentation and homolytic dissociation were strongly suggested as the initial reaction step for β-O-4 compounds and α-O-4 compounds, respectively. The difference in reaction pathway between compounds with different linkages was believed to result from thermodynamics of the radical initiation. The rate constants for the different reaction pathways were predicted from ab initio density functional theory calculations and pre-exponential literature values. The computational findings were consistent with the experiment results, further supporting the different pyrolysis mechanisms for the β-ether linked and α-ether linked compounds. A combination of the two pathways from the dimeric model compounds was able to describe qualitatively the pyrolysis of a trimeric lignin model compound containing both β-O-4 and α-O-4 linkages.

 Please wait while we load your content...
Please wait while we load your content...